Several techniques and drug regimens have been tried from time to time to eliminate the anxiety and to prolong the intra and post-operative analgesia during regional anaesthesia, with partial or greater success. Alpha 2 adrenergic agonists have analgesic property and potentiate the effect of local anaesthetics and prolong the duration of both motor, sensory spinal blockade and post-operative analgesia.
Clonidine is a selective partial agonist for alpha 2 adrenoceptors. It is used increasingly as adjuvant to prolong bupivacaine spinal anaesthesia [1-3]. In addition to its intrathecal route, its use in caudal analgesia [4] and through intravenous route [5] is also documented. Thus its efficacy as an analgesic and its synergistic action with spinal bupivacaine has been clearly established.
Dexmedetomidine is a selective α2-adrenoceptor agonist with an α2/α1 selectivity ratio eight to ten times higher than that of clonidine (1600:1 as compared to 200:1 for clonidine) [6,7]. Thus recently, it has become a mainstay drug in anaesthetic procedures [8]. This noble drug dexmedetomidine was approved by Food and Drug Administration only as a short term (<24 hours) sedative for mechanically ventilated adult patients. However, it is now used outside intensive care unit settings.
Hall JE et al., concluded that intravenous dexmedetomidine has analgesic, sedative and amnestic properties [8]. It also reduces the requirement of post-operative analgesics [9-11]. It suppresses the haemodynamic response to endotracheal intubation [12-14]. In addition to these, dexmedetomidine also decreases the plasma catecholamine level [15]. When used as supplement in spinal anaesthesia, its ability to prolong the sensory and motor block was comparable with fentanyl [16]. It is well documented being as adjuvant to local anaesthetics during epidural anaesthesia [17-19].
Since clonidine has been proven clinically to prolong spinal bupivacaine analgesia when used intravenously, it was hypothesised that dexmedetomidine being an alpha-2 agonist belonging to the same group as clonidine will similarly prolong duration of spinal bupivacaine analgesia. Moreover, there are only a few studies of intravenous dexmedetomidine for prolongation of spinal bupivacaine analgesia. For e.g., Kanazi GE et al., concluded that dexmedetomidine and clonidine when added to intrathecal bupivacaine produced similar prolongation in the duration of spinal anaesthesia with preserved haemodynamic stability and lack of sedation [1]. Similarly, El-Hennawy Am et al., concluded that children undergoing lower abdominal surgeries with no advantage of dexmedetomidine over clonidine can have prolonged analgesic effect on addition of dexmedetomidine or clonidine to caudal bupivacaine anaesthesia [4].
This present study aimed to prove the hypothesis that dexmedetomidine being a more selective alpha 2 agonist than clonidine should provide longer duration of block along with effective intraoperative sedation by its action on the locus ceruleus.
This study was designed to evaluate and compare the efficacy of intravenous dexmedetomidine with clonidine on the duration of spinal blockade and post-operative analgesia as premedication to intrathecal bupivacaine. The study also compares the characteristics of the spinal and motor blockade provided by these two drugs, their side-effects and the level of sedation.
Materials and Methods
The randomised controlled trial was conducted from 1/5/2019 to 31/11/2019 in Datta Meghe Institute of Medical Sciences, Sawangi, Wardha, Maharashtra, India. The present study was approved by the Institutional Ethics Committee (DMIMS/IEC/2019/7941) and written informed consent was taken from each patient prior to the study.
Sample size was derived using software openepi.com. Assuming the average onset of sensory block of 2.91 minutes and standard deviation of 1.16 minutes [21]. Keeping power at 80% with confidence interval of 95% (alpha error at 0.05), a sample size of 44 patients in each group were required to detect a minimum of 20% difference in the mean onset of sensory block. Considering the dropouts, 50 patients per study group were taken.
A total of 100 patients aged 20-60 years, American Society of Anaesthesiologists physical Status I or II, both sex, weighing 40-80 kg, measuring 140 to 180 cm in height with BMI 20-25 kg/m2 scheduled for elective surgery under spinal anaesthesia were included in this study. Exclusion criteria were unwilling patient, patient with history of known allergies to study drugs, patients undergoing emergency surgeries, patients with ASA Class III or higher, cardiovascular diseases and severe respiratory diseases, systemic illness such as hypertension, diabetes, hepatic failure, renal failure, hyperthyroidism and endocrine disorders, alcohol or drug abuse, psychological disorders, spinal deformities, contra-indications to spinal anaesthesia e.g., coagulation defect, infection of puncture site, pre-existing neurological deficits in the body.
Pre-anaesthetic evaluation of all patients including detailed history, physical examination, systemic examination, routine investigations were done. All patients were kept nil by mouth six hours before surgery.
On arrival to the operation theatre on the day of surgery, an 18 G iv cannula was secured and patients received 500 mL ringer’s lactate solution before spinal anaesthesia. Routine monitoring devices like pulse-oximeter, non-invasive BP, ECG monitor were applied. Heart rate, SBP, DBP and MAP were recorded and were considered as baseline values.
The study drugs were prepared by a blinded anaesthesiologist before the surgery. The drugs were diluted to a total volume of 10 mL in a 10 cc syringe. Blinding was done by excluding the anaesthesiologist who prepared the study drugs during the administration of the drugs and spinal anaesthesia. The patients were also not involved in group allocation. Patients were randomly allocated into two groups of 50 patients each (Group A and B) using computer generated random number tablets and allocation of same sealed envelope technique to receive the study drugs. Group A received intravenous dexmedetomidine and Group B received intravenous clonidine.
The study drugs were administered intravenously over a period of 10 minutes by the researcher. Five minutes after administration of the study drug, the patient was put in left lateral position. Spinal analgesia was given at L3-L4 space by midline approach using a 25 gauge Quinke spinal needle. A 0.5% hyperbaric bupivacaine 15 mg was injected intrathecally and patient was put in supine position.
The blinded anaesthesiologist recorded the vitals such as HR, MAP, SPO2, RR, 2 minutes after pre-medication, immediately before or 60 seconds after dural puncture and every 10 minutes after during surgery. Fall of systolic blood pressure below 20% of the baseline was corrected by fluids or vasoconstrictors. Bradycardia was treated with injection atropine.
Sensory blockade was assessed by the blinded anaesthesiologist using a gentle pin prick with a sterile needle to check the level of blockade achieved and duration was defined as the time for sensory block to regress to L5-S2 dermatome. Motor block was assessed using a modified Bromage scale (Grade 0- no paralysis, 1- inability to raise extended leg, 2- inability to flex the knee, 3- inability to flex the ankle). Recovery time for the sensory blockade was defined as two dermatome regression of anaesthesia from the maximum level. Motor block duration was the time to return to Grade 1 on the modified Bromage scale. Pain was assessed using the visual analogue scale (VAS 0: no pain; 10: worst possible pain). Patients with a VAS score of 4 or more were administered injection diclofenac sodium 75 mg, intramuscularly. The time of first request for intraoperative analgesic was recorded as the duration of analgesia.
The Ramsay sedation score was recorded every 10 minutes throughout intraoperative period to assess the level of sedation (1-anxious, agitated or restless or both, 2-cooperative, oriented and tranquil, 3-responds to command, 4-asleep but with brisk response to glabellar tap, 5-asleep with sluggish response to glabellar tap or loud auditory stimulus, 6-asleep with no response) [Table/Fig-1].
Statistical Analysis
Statistical Package for Social Sciences (SPSS Inc. Chicago, IL, USA) version 20.0 software was used for analysis. The parameters and patient data were recorded and entered in Microsoft Excel sheet. The data were analysed using analysis of variance or chi-square test. p-value <0.05 considered statistically significant.
Results
The demographic profiles in terms of age, weight, height and BMI of the two groups were compared and no statistically significant difference was observed between the two groups [Table/Fig-2].
| Patient profile | Group A (dexmedetomidine) | Group B (clonidine) | p-value |
|---|
| Age (years) | 45.6±6.9 | 45.7±7.3 | 0.944 |
| Weight (kg) | 56.3±8.7 | 57.2±6.5 | 0.559 |
| Height (cm) | 159±5.5 | 158±4.4 | 0.317 |
| BMI (kg/m2) | 21.86±1.84 | 21.42±1.52 | 0.195 |
Intravenous dexmedetomidine during bupivacaine spinal anaesthesia resulted in earlier onset of sensory block as compared to clonidine. Dexmedetomidine also achieved maximum sensory level of block (T4-T5) as compared to clonidine (T6-T8). Dexmedetomidine reduce the time of onset and also increased the time to first rescue analgesia of motor block compared to clonidine [Table/Fig-3].
Time of onset of sensory and motor block, highest t sensory level and post-operative analgesia.
| Variables | Group A (dexmedetomidine) | Group B (clonidine) | p-value |
|---|
| Time of onset of sensory block (minutes) | 2.60±1.12 | 3.45±1.50 | 0.0018* |
| Time for two segment regression of sensory block (minutes) | 150.34±20 | 128.48±16 | 0.0031* |
| Time for regression of motor block to Bromage scale 1 (minutes) | 148.56±40 | 144±38 | 0.6769 |
| Highest sensory level (segments) | T4-T5 | T6-T8 | |
| Time of first request of analgesic (minutes) | 250.46±52.10 | 180.76±50.28 | <0.001* |
*S: Significant
Patients with sedation scores >3 were 52% in Group A (receiving dexmedetomidine) and 32% in Group B (receiving clonidine). Thus, better sedation was achieved with dexmedetomidine than clonidine [Table/Fig-4].
| Sedation scores during surgery | Group A (dexmedetomidine) | Group B (clonidine) | p-value |
|---|
| 1 | 8 | 12 | 0.31 |
| 2 | 2 | 4 | 0.709 |
| 3 | 14 | 18 | 0.73 |
| 4 | 26 | 16 | 0.042* |
| 5 | 0 | 0 | |
*S: Significant
The occurrence of bradycardia and hypotension was more in Group A receiving dexmedetomidine as compared to Group B receiving clonidine, but it was not significant. Nausea and vomiting were seen more in Group B but was not significant [Table/Fig-5].
| Variables | Group A (dexmedetomidine) | Group B (clonidine) | p-value |
|---|
| Bradycardia | 12 | 7 | 0.20 |
| Hypotension | 16 | 12 | 0.37 |
| Nausea and vomiting | 4 | 10 | 0.08 |
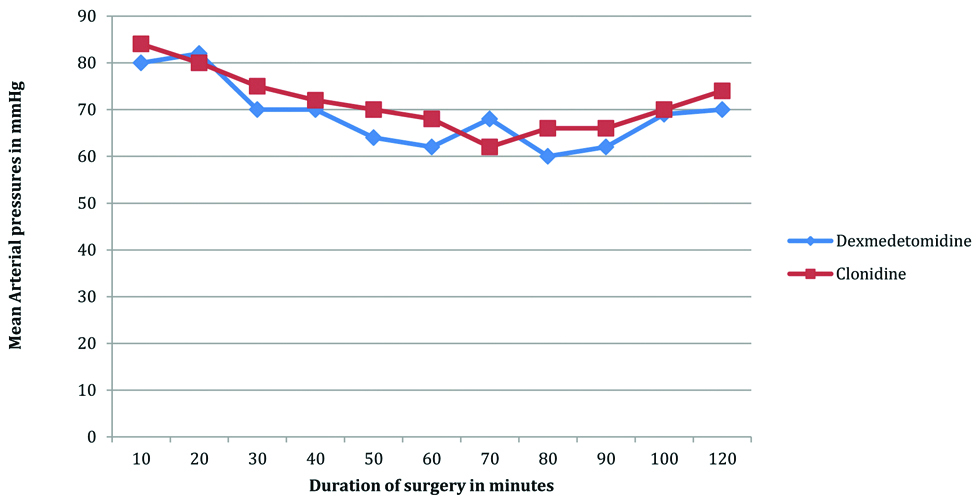
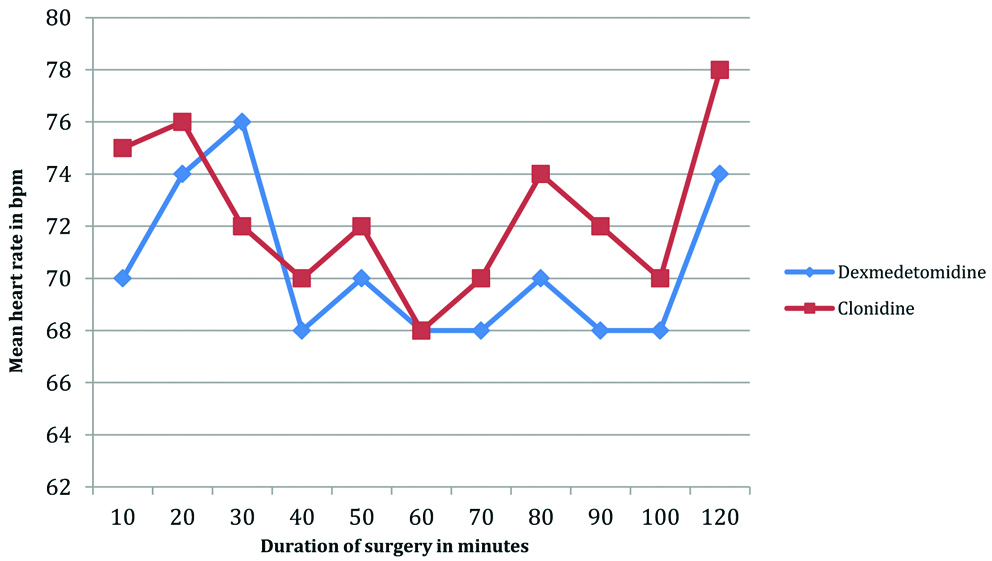
Lower mean arterial pressures and the mean heart rates were observed in Group A (dexmedetomidine) as compared to Group B (clonidine) [Table/Fig-6,7].
Comparison between mean arterial pressures in dexmedetomidine and clonidine group during intaraoperative period.
Comparison of the heart rate in the dexmedetomidine and clonidine groups in the intraoperative period.
Discussion
Effectiveness of alpha 2 agonists as adjuvant to intrathecal bupivacaine in prolonging the duration of block is well documented. It has been proved that intravenous clonidine prolongs spinal bupivacaine analgesia. A hypothesis was framed that dexmedetomidine being an alpha 2 agonist belonging to the same group as clonidine and having nearly eight to ten times the selectivity for alpha 2 adrenergic receptors should prolong spinal bupivacaine analgesia more than clonidine when given by intravenous route. It may be stated that dexmedetomidine given intravenously reaches the spinal cord in a concentration to act as an adjuvant to intrathecal bupivacaine.
In the present study, authors have used a dose of 0.5 μg/kg iv dexmedetomidine in conjunct with Jaakola ML et al., who demonstrated moderate analgesia with a dose of 0.5 μg/kg iv dexmedetomidine without adverse-effects of bradycardia and hypotension [20]. A dose of 1.0 μg/kg iv of clonidine was selected based on the study conducted by Kaabachi O et al., who observed that clonidine is a safe and effective adjuvant to bupivacaine in spinal anaesthesia at a dose of 1 μg/kg iv [2].
It was found that there was a statistically significant longer sensory and motor block in dexmedetomidine group than in the clonidine group. The analgesia produced by alpha 2 agonists is due to their action on the alpha 2 receptors located in the brainstem and spinal cord. This action could explain the prolongation of spinal anaesthesia after intravenous administration of dexmedetomidine and clonidine.
In the present study, two segment regression time of sensory block and the time for the first rescue analgesia was longer in the dexmedetomidine group. The present results matched the findings of Reddy VS et al., [21]. This is most probably because dexmedetomidine has eight to ten times more affinity to Alpha 2 adrenoceptors (especially for Alpha 2A and Alpha 2C subtypes) compared to clonidine [22]. There was no difference in the time for regression of motor block among the groups. Reddy VS et al., reported similar findings i.e., no effect of alpha 2 agonists on motor block, when comparing iv dexmedetomidine (0.5 μg/kg) and iv clonidine (1.0 μg/kg) as adjuvants to intrathecal bupivacaine [21]. These observations correlate with the finding that the 50% effective concentration of clonidine required to block motor fibres is approximately 4 fold that of pain conducting small, unmyelinated C fibers [23]. Similar mechanisms can explain the prolongation of sensory block as compared with motor block by dexmedetomidine whose mechanism of action is similar to clonidine.
An initial transient increase in blood pressure was attributed to the direct effects of alpha 2 adrenoceptor stimulation of vascular smooth muscle. After this transient increase, there was a reduction in blood pressure due to both centrally and peripherally mediated sympatholytic action. Haemodynamic changes were more in the dexmedetomidine group but statistically insignificant.
Patients in the dexmedetomidine group were sedated properly with 52% attaining Ramsay sedation score >3 as compared to 32% in the clonidine group and was found to be statistically significant. A study conducted by Hall JE et al., reported minimal or no respiratory depression following intravenous dexmedetomidine, which has also been confirmed by the results of the present study. The alpha 2 agonists cause sedation by acting on the alpha 2 receptors (2b and 2c subtypes) in the brain and spinal cord. The alpha 2 receptors inhibit adenylyl cyclase which in turn decreases the levels of cyclic Adenosine Monophosphate (cAMP) causing hyperpolarisation of noradrenergic neurons in the locus ceruleus [24]. This causes inhibition of noradrenaline release and suppression of neuronal activity of ascending noradrenergic pathways resulting in hypnosis and sedation [25].
Limitation(s)
The main limitation in the present study was the absence of standardised preloading or co-loading technique.
Conclusion(s)
The results obtained in the study clearly demonstrate that intravenous dexmedetomidine results in an early onset of action of bupivacaine anaesthesia, rapid onset of both sensory and motor block and prolonged duration of analgesia in the post-operative period as compared to intravenous clonidine. Thus, the present authors would recommend intravenous dexmedetomidine as an adjuvant in cases undergoing elective surgeries under bupivacaine spinal anaesthesia although it is associated with a few haemodynamic side-effects like bradycardia and hypotension which can be prevented by administering iv glycopyrrolate or iv atropine and proper preloading with iv fluids, respectively.
*S: Significant
*S: Significant