Comparative Study of Ondansetron and Palonosetron for Prevention of Nausea and Vomiting Following Upper Abdominal Surgeries under General Anaesthesia: A Randomised Control Trial
Deepak Premnarayan Gupta1, Vijay Chandak2
1 Junior Resident, Department of Anaesthesia, DMIMS, Sawangi, Wardha, Maharashtra, India.
2 Associate Professor, Department of Anaesthesia, DMIMS, Sawangi, Wardha, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Deepak Premnarayan Gupta, Junior Resident, Department of Anaesthesia, DMIMS, Wardha-442004, Maharashtra, India.
E-mail: deardeepu2589@gmail.com
Introduction
Postoperative Nausea and Vomiting (PONV) is a common entity following surgical procedure. It may result into serious complication like aspiration of gastric contents, prolonged recovery period. Palonosetron is a selective serotonin antagonist that is 5HT3 receptor antagonist with little to no affinity for other receptors and has a longer duration of action. Ondansetron is also a 5HT3 receptor antagonist with shorter duration of action and some side effects.
Aim
To compare the effectiveness of ondansetron and palonosetron for the prevention of PONV following upper abdominal surgeries.
Materials and Methods
This prospective single-blind study included 120 patients randomly assigned to the palonosetron group (n=60) or the ondansetron group (n=60). Using the chi-square test and calculating p-value, the two groups were compared.
Results
The incidence of nausea, vomiting and use of rescue antiemetic was significantly less in palonosetron group as compared to ondansetron group.
Conclusion
From the study, it can be concluded that palonosetron at a dose of 0.075 mg is safe, with lesser side effects and proved more effective than ondansetron 4 mg in prevention of PONV.
Adverse effect, Antiemetic, Postoperative
Introduction
Postoperative Nausea and Vomiting (PONV) is defined as nausea, retching, or vomiting occurring in Post Anaesthesia Care Unit (PACU) or 24-hours following a surgical procedure [1]. PONV are common problems of general as well as regional anaesthesia and a leading cause of delayed discharge and unanticipated hospital admission after surgical procedure. Overall incidence is 30%, but in certain high risk patients it can be as high as 80% [2]. Various studies have already been done and suggest wide variation in overall incidence [3-6]. PONV is frequent in abdominal surgery. Hence, the use of potent antiemetic becomes important to treat it effectively [7].
A study by Apfel CC et al., described nausea as the desire to vomit without the presence of expulsive muscular movements [8,9]. Vomiting is described as pre-set events of motor and autonomic response that results in forceful expulsion of gastric content through the mouth as described by the study of Hasler WL and Chey WD [10]. Retching is the term used to describe the labored, rhythmic respiratory activity, and abdominal musculature contractions that usually precedes vomiting as described in the study of Hasler WL and Chey WD [10]. Retching along with expulsion of gastric content is counted as vomiting [11].
Palonosetron is a second generation serotonin 5HT3 receptor antagonist. Palonosetron exhibited allosteric binding to 5HT3 receptor [12]. It also inhibit neurokinin-1 receptor and produces antiemetic property [13].
A 5HT3 receptors antagonist is used to prevent PONV in the patients undergoing abdominal surgeries under general anaesthesia. FDA has approved the use of Palonosetron for prophylaxis of PONV in 2008.
Palonosetron has an indirect effect by its allosteric binding with 5HT3 receptors [14]. This may be the clinical site of action of the 5HT3 receptor antagonists. Half-life of ondansetron is 3.5 to 5.5 hours and that of palonosetron is 40 hours [15]. IV palonosetron 0.075 mg is found to be more potent than 0.025 mg and 0.050 mg in preventing PONV [16,17].
Many studies have been conducted to evaluate safety and efficacy of antiemetics in a specific groups undergoing particular type of surgery but there are insufficient data available on upper abdominal surgeries cases. To bridge this gap this randomised single blind study was conducted to compare the antiemetic effect and assess the safety of palonosetron (750 mcg) against ondansetron (4 mg) on population covering all eligible postoperative candidates undergoing general anaesthesia with upper abdominal surgeries.
The novelty of the study is that it will further strengthen the hypothesis regarding effect of palonosetron drug in upper abdominal surgeries which has been compared with the popular used intravenous antiemetic ondansetron.
Materials and Methods
Present randomised single blind study was conducted on 120 patients between 18 to 65 years of age, who were scheduled for abdominal surgeries under general anaesthesia, in Jawaharlal Nehru Medical College, Acharya Vinoba Bhave Rural Hospital, Sawangi, Wardha, Maharashtra, India, between August 2018 to August 2019.
Sample size was derived using software openepi.com. For the sample size of the two groups, the power was set at 80% (β=0.2) with a 30% reduction of PONV incidence. The significant level was set as 5% (α=0.05, two-tailed). The calculated sample size was minimum 42, so taking potential drop-outs into consideration; the sample size was set as 60 for each group.
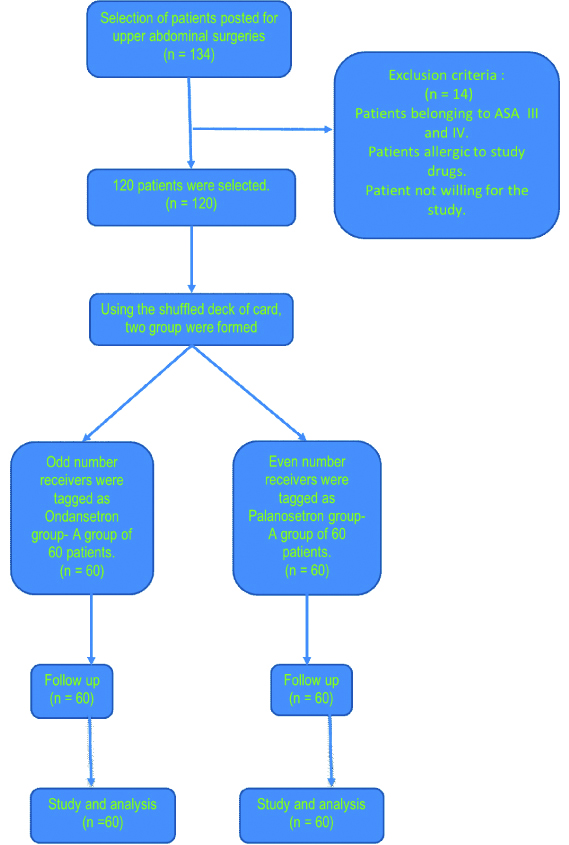
After getting ethical committee approval letter {RefNo. DMIMS(DU)/IEC/2019/7947}, patients were selected randomly after taking informed written consent. After selection, patient was randomly allocated into two groups, 60 in each, by card sampling. Using the shuffled deck of cards, even number cards were tagged for one group and odd number cards were tagged for other group. Group I received inj ondansetron (4 mg) while group II received inj palonosetron (0.075 mg). Both drugs were given postoperatively before extubation.
Inclusion/Exclusion Criteria
Patients with ASA class I and II, age between 18 to 65 years, weight between 50-80 kg were included. Patients belonging to ASA III and IV, allergic to study drugs were excluded.
A study medication (2 mL) was prepared by one of the investigators and was administered. Thus, two groups of 60 patients each were formed where the researchers were unaware of the group distributed to him/her. Group A (n=60) received ondansetron 2 mL (4 mg), and group B (n=60) received 0.075mg of palonosetron, 2 minutes before injecting reversal. The anaesthetic regimen and surgical procedure was standardised for all patients. Premedication with opioid and sedation (midazolam) and glycopyrolate was given. Intravenous propofol 2 mg/kg was given to patient and anaesthesia was induced. For intubation 0.1 mg/kg vecurnium was used. Anaesthesia was maintained with sevoflurane. Ventilation was controlled mechanically. At the end of surgery after stopping sevoflurane and nitrous oxide, i.v inj. palonosetron (750 mcg) or inj ondansetron (4 mg) was given. Patient was reversed with neostigmine 2.5 mg and glycopyrolate 0.5 mg to reverse residual neuroparalytic block. Tracheal tube was removed after complete reversal and clearing the throat by suction. After surgery, the patients were sent to PACU. Blood pressure, heart rate, and respiratory rate were monitored. Emetic episodes were assessed immediately after operation and at 1 hour interval for 24 hours. Patients with complain of nausea, vomiting, or retching were administered injection dexamethasone 8 mg as a rescue antiemetic [Table/Fig-1].
Statistical Analysis
The parameters and patient data were recorded and entered in Microsoft Excel sheet. (SPSS Inc. Chicago, IL, USA) version 20.0 software for windows was used for analysis. Incidence of PONV were compared in two study groups and the results were analysed by using chi-square test. The p-value <0.05 was considered to be significant.
Results
All patients were comparable according to gender, age, bodyweight, duration of anaesthesia and surgery [Table/Fig-2].
Demography and duration of procedure.
| Gender | Group A Ondansetron | Group B Palonosetron | p-value |
|---|
| Male | 58 | 63 | 0.60 |
| Female | 62 | 57 | 0.60 |
| Age (Yrs.) | 34.7±10.3 | 36.36±10.44 | 0.38 |
| Wt. (KG) | 66±10 kg | 68±8 kg | 0.22 |
| Duration of anaesthesia | 120±45 min | 130±30 min | 0.15 |
| Duration of surgery | 120±45 min | 130±30 min | 0.15 |
[Table/Fig-3] represent the distribution of open surgeries and laparoscopic surgeries in both groups. There was no significant difference among the two groups.
| Type of surgery | Group A Ondansetron | Group B Palonosetron | p-value |
|---|
| Open surgery | 32 | 36 | 0.58 |
| Laparoscopic surgery | 28 | 24 | 0.58 |
Open surgeries includes appendectomy, exploratory laparotomy, hemi colectomy, bilateral herniotomy, and cholecystectomy; Laparoscopic surgeries include laparoscopic cholecystectomy, laparoscopic appendectomy, diagnostic laparoscopy, laparoscopic urolithotomy
Incidence of nausea was assessed at the interval of 6 hour up to 24 hours after surgery. Incidence of nausea was found to be significant in first 6 hours, in between 12 to 18 hours among the two groups [Table/Fig-4].
Incidence of nausea in 24 hours.
| Time | Group 1 Ondansetron | Group 2 Palonosetron | p-value |
|---|
| 0-6 hrs | 7 | 0 | 0.0194 (significant) |
| 6-12 hrs | 4 | 1 | 0.360 |
| 12-18 hrs | 6 | 0 | 0.0362 (significant) |
| 18-24 hrs | 3 | 1 | 0.611 |
| Total | 20 | 2 | 0.0001 (significant) |
Retching was significant in first 6 hours and in between 12 to 18 hours among the two groups [Table/Fig-5].
Incidence of retching in 24 hours.
| Time | Group 1 Ondansetron | Group 2 Palonosetron | p-value |
|---|
| 0-6 hrs | 6 | 0 | 0.0362 (Significant) |
| 6-12 hrs | 3 | 0 | 0.242 |
| 12-18 hrs | 7 | 0 | 0.0194 (Significant) |
| 18-24 hrs | 4 | 1 | 0.360 |
| Total | 20 | 1 | 0.000185 (Significant) |
Vomiting was significant in first 6 hours and in between 12 to 18 hours among the two groups [Table/Fig-6].
Incidence of vomiting in 24 hours.
| Time | Group 1 Ondansetron | Group 2 Palonosetron | p-value |
|---|
| 0-6 hrs | 6 | 0 | 0.0362 (significant) |
| 6-12 hrs | 2 | 0 | 0.4758 |
| 12-18 hrs | 7 | 0 | 0.0194 (significant) |
| 18-24 hrs | 6 | 1 | 0.1192 |
| Total | 21 | 1 | 0.0001 (significant) |
No significant differences were observed between the groups in adverse effects such as headache, itching and allergic reaction during the first 24 hours after surgery. The p-value remains insignificant as described in [Table/Fig-7].
The incidence of adverse effects in each group.
| Adverse effect in each group | Group A (Ondansetron) | Group B (Palonosetron) | p-value |
|---|
| Headache | 6 | 4 | 0.1 (Not significant) |
| Itching | 2 | 1 | 1 (Not significant) |
| Allergic reaction | 1 | 0 | 1 (Not Significant) |
Severity of postoperative nausea was found to be effective (p-value=0.03) between 12 to 18 hours while severity of postoperative vomiting was found to be effective (p-value=0.01) in between 12 to 18 hours. Overall, the two groups do not have significant differences in reducing the severity of PONV [Table/Fig-8,9].
Scoring system for nausea and vomiting [18].
| Score | Nausea | Vomiting |
|---|
| 0 | None | None |
| 1 | Mild intermittent nausea | One episode |
| 2 | Moderate constant nausea | Several episode |
| 3 | Severe nausea | Continuous episode |
PONV score according to duration.
| Postoperative nausea score | Postoperative vomiting score |
|---|
| Duration | Nausea score | Ondansetron group | Palonosetron group | p-value | Duration | Vomiting score | Ondansetron group | Palonosetron group | p-value |
|---|
| 0-6 hrs. | 0 | 53 | 60 | 0.01* | 0-6 hrs. | 0 | 54 | 60 | 0.03* |
| 1 | 5 | 0 | 0.06 | | 1 | 5 | 0 | 0.06 |
| 2 | 0 | 0 | 0.92 | | 2 | 0 | 0 | 0.92 |
| 3 | 2 | 0 | 0.47 | | 3 | 1 | 0 | 1.0 |
| 6-12 hrs. | 0 | 56 | 59 | 0.36 | 6-12 hrs. | 0 | 58 | 60 | 0.47 |
| 1 | 3 | 1 | 0.61 | | 1 | 2 | 0 | 0.47 |
| 2 | 0 | 0 | 0.92 | | 2 | 0 | 0 | 0.92 |
| 3 | 1 | 0 | 1.0 | | 3 | 0 | 0 | 0.92 |
| 12-18 hrs. | 0 | 54 | 60 | 0.03* | 12-18 hrs. | 0 | 53 | 60 | 0.01* |
| 1 | 6 | 0 | 0.03* | | 1 | 7 | 0 | 0.01* |
| 2 | 0 | 0 | 0.92 | | 2 | 0 | 0 | 0.92 |
| 3 | 0 | 0 | 0.92 | | 3 | 0 | 0 | 0.92 |
| 18-24 hrs. | 0 | 57 | 59 | 0.6 | 18-24 hrs. | 0 | 54 | 59 | 0.11 |
| 1 | 3 | 1 | 0.6 | | 1 | 6 | 1 | 0.11 |
| 2 | 0 | 0 | 0.92 | | 2 | 0 | 0 | 0.92 |
| 3 | 0 | 0 | 0.92 | | 3 | 0 | 0 | 0.92 |
The symbol “*” denotes the significant difference among the two groups
Blood pressure and pulse rate were noted among ondansetron and palonosetron group. The p-value was insignificant for ondansetron and palonosetron group (p >0.05) as shown in the [Table/Fig-10].
Comparison of haemodynamic variation among groups.
| Variables | Ondansetron | Palonosetron | p-value |
|---|
| Mean | Standard deviation | Mean | Standard deviation |
|---|
| Systolic blood pressure | 107.705 | 11.014 | 106.393 | 11.551 | 0.525 |
| Diastolic blood pressure | 76.885 | 11.334 | 75.573 | 12.045 | 0.540 |
| Pulse rate | 80.918 | 5.532 | 80.754 | 6.168 | 0.153 |
Discussion
In the present study, the antiemetic drugs (ondansetron and palonosetron) were given to the patients just prior to the extubation. Postoperatively, the haemodynamic parameters were noted among the two groups (ondansetron and palonosetron group). It was found that there was no significant difference between the mean values of measured haemodynamic parameters in two study groups [Table/Fig-10]. This observation was similar to study conducted by Paventi S et al., [19].
This study showed that palonosetron was well tolerated and found to be clinically effective in terms of retching, nausea and vomiting (p<0.05) [Table/Fig-4,5 and 6]. Recently, there have been studies comparing the effects of palonosetron and other 5HT3 receptor antagonists for the prevention of PONV, sharing similar findings with present study [3,20,21]. The study done by Singhal DM and Sharma N demonstrate that overall incidence of PONV were less as compared to other antiemetic drugs [22,23]. The study done by Moon YE et al., also states that palonosetron is a better drug than ondansetron in preventing PONV [24]. The present study support strongly to the evidence that palonosetron has a potent antiemetic activity within first 6 hours. No significant difference was found between ondansetron and palonosetron in reducing the severity of nausea and vomiting [Table/Fig-9]. This finding was supported by the study conducted by Aydin A et al., [3]. Also, there was no notable difference in side effects among both the groups. Similar finding are found in the study conducted by De Leon A where they compared the adverse effects of ondansetron, palonosetron and dolasetron and stated that adverse reaction were similar in all three groups [25].
The present study also showed that p-value for ondansetron and palonosetron group is highly significant in terms of preventing nausea. This finding of the study is also supported by the study Naguib M et al. [26]. Aydin A et al. studied and compared ondansetron, palonosetron and tropisetron for preventing PON, PONV. As stated in their study, the incidence of PONV was less in palonosetron group [3].
Limitation(s)
Equipotent doses were not used. Instead optimal dose were used for comparison. Also the cost of the palonosetron was much more as compared to ondansetron in Indian market. Many corporate hospitals can afford to use the drug palonosetron instead of ondansetron. But the government institutions of developing countries can find it difficult to use the palonosetron.
Conclusion(s)
In conclusion, bolus of intravenous palonosetron 0.075 mg was found to be more efficacious than bolus of intravenous ondansetron 4 mg especially during the first six hours.
Open surgeries includes appendectomy, exploratory laparotomy, hemi colectomy, bilateral herniotomy, and cholecystectomy; Laparoscopic surgeries include laparoscopic cholecystectomy, laparoscopic appendectomy, diagnostic laparoscopy, laparoscopic urolithotomy
The symbol “*” denotes the significant difference among the two groups
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Dec 21, 2019
Manual Googling: Feb 10, 2020
iThenticate Software: Feb 28, 2020 (18%)
[1]. Collins A, Postoperative nausea and vomiting in adults: Implications for critical careCrit Care Nurse 2011 31:36-45.10.4037/ccn201147022135330 [Google Scholar] [CrossRef] [PubMed]
[2]. Pierre S, Whelan R, Nausea and vomiting after surgeryContin Educ Anaesthesia, Crit Care Pain 2013 13(1):28-32.10.1093/bjaceaccp/mks046 [Google Scholar] [CrossRef]
[3]. Aydin A, Kaçmaz M, Boyaci A, Comparison of ondansetron, tropisetron, and palonosetron for the prevention of postoperative nausea and vomiting after middle ear surgeryCurr Ther Res-Clin Exp [Internet] 2019 91:17-21.Available from: https://doi.org/10.1016/j.curtheres.2019.06.00210.1016/j.curtheres.2019.06.00231384338 [Google Scholar] [CrossRef] [PubMed]
[4]. Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, Consensus guidelines for the management of postoperative nausea and vomitingAnesth Analg 2014 118(1):85-113.10.1213/ANE.000000000000000224356162 [Google Scholar] [CrossRef] [PubMed]
[5]. Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N, A simplified risk score for predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centersAnesthesiology 1999 91(3):693-700.10.1097/00000542-199909000-0002210485781 [Google Scholar] [CrossRef] [PubMed]
[6]. McCracken G, Houston P, Lefebvre G, Guideline for the management of postoperative nausea and vomitingJ Obstet Gynaecol Can 2008 30(7):600-607.:608-16.10.1016/S1701-2163(16)32895-X [Google Scholar] [CrossRef]
[7]. Muchatuta NA, Paech MJ, Management of postoperative nausea and vomiting: Focus on palonosetronTher Clin Risk Manag 2009 5(1):21-34.10.2147/TCRM.S3437 [Google Scholar] [CrossRef]
[8]. Apfel CC, Roewer N, Korttila K, How to study postoperative nausea and vomitingActa Anaesthesiol Scand 2002 46(8):921-28.10.1034/j.1399-6576.2002.460801.x12190791 [Google Scholar] [CrossRef] [PubMed]
[9]. Gan TJ, Risk factors for postoperative nausea and vomitingAnesth Analg 2006 102(6):1884-98.10.1213/01.ANE.0000219597.16143.4D16717343 [Google Scholar] [CrossRef] [PubMed]
[10]. Hasler WL, Chey WD, Nausea and VomitingGastroenterology 2003 125(6):1860-67.10.1053/j.gastro.2003.09.04014724837 [Google Scholar] [CrossRef] [PubMed]
[11]. Soto E, Berger AM, Nausea and vomitingPalliat Care 2011 :115-28.10.1016/B978-1-4377-1619-1.00008-1 [Google Scholar] [CrossRef]
[12]. Rojas C, Stathis M, Thomas AG, Massuda EB, Alt J, Zhang J, Palonosetron exhibits unique molecular interactions with the 5-HT3 receptorAnesth Analg 2008 107(2):469-78.10.1213/ane.0b013e318172fa7418633025 [Google Scholar] [CrossRef] [PubMed]
[13]. Rojas C, Li Y, Zhang J, Stathis M, Alt J, Thomas AG, The antiemetic 5-HT3 receptor antagonist Palonosetron inhibits substance P-mediated responses in vitro and in vivoJ Pharmacol Exp Ther 2010 335(2):362-68.10.1124/jpet.110.16618120724484 [Google Scholar] [CrossRef] [PubMed]
[14]. Bunce KT, Tyers MB, The role of 5-HT in postoperative nausea and vomitingBr J Anaesth 1992 69(7 Suppl 1):60S-62S.10.1093/bja/69.supplement_1.60S1486015 [Google Scholar] [CrossRef] [PubMed]
[15]. Ho KY, Gan TJ, Pharmacology, pharmacogenetics, and clinical efficacy of 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomitingCurr Opin Anaesthesiol 2006 19(6):606-11.10.1097/01.aco.0000247340.61815.3817093363 [Google Scholar] [CrossRef] [PubMed]
[16]. Kovac AL, Eberhart L, Kotarski J, Clerici G, Apfel C, A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo in preventing postoperative nausea and vomiting over a 72-hour periodAnesth Analg 2008 107(2):439-44.10.1213/ane.0b013e31817abcd318633021 [Google Scholar] [CrossRef] [PubMed]
[17]. Candiotti KA, Kovac AL, Melson TI, Clerici G, Joo Gan T, A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo for preventing postoperative nausea and vomitingAnesth Analg 2008 107(2):445-51.10.1213/ane.0b013e31817b5ebb18633022 [Google Scholar] [CrossRef] [PubMed]
[18]. Sharma S, Khanna S, Das J, Mehta Y, Handa KK, A randomized study to compare palonosetron with ondansetron for prevention of postoperative nausea and vomiting following middle ear surgeriesJ Anaesthesiol Clin Pharmacol 2019 35(2):182-87.10.4103/joacp.JOACP_196_1731303706 [Google Scholar] [CrossRef] [PubMed]
[19]. Paventi S, Santevecchi A, Ranieri R, Efficacy of a single-dose ondansetron for preventing post-operative nausea and vomiting after laparoscopic cholecystectomy with sevoflurane and remifentanil infusion anaesthesiaEur Rev Med Pharmacol Sci 2001 5(2):59-63. [Google Scholar]
[20]. Kim YY, Moon SY, Song DU, Lee KH, Song JW, Kwon YE, Comparison of palonosetron with ondansetron in prevention of postoperative nausea and vomiting in patients receiving intravenous patient-controlled analgesia after gynecological laparoscopic surgeryKorean J Anesthesiol [Internet]. 2013/02/15 201 64(2):122-26.Available from: https://www.ncbi.nlm.nih.gov/pubmed/2345949910.4097/kjae.2013.64.2.12223459499 [Google Scholar] [CrossRef] [PubMed]
[21]. Park SK, Cho EJ, A randomized, double-blind trial of palonosetron compared with ondansetron in preventing postoperative nausea and vomiting after gynaecological laparoscopic surgeryJ Int Med Res 2011 39(2):399-407.10.1177/14732300110390020721672343 [Google Scholar] [CrossRef] [PubMed]
[22]. Singhal DM, Comparison of intravenous ondansetron and palonosetron in prevention of postoperative nausea and vomiting in patients posted for elective laproscopic cholecystectomyJ Med Sci Clin Res 2018 6(4):10-17.10.18535/jmscr/v6i4.02 [Google Scholar] [CrossRef]
[23]. Sharma N, Bhargava M, Chaudhary V, Sharma D, Mishra A, Chaudhary PK, Advani UKS, Comparison of the efficacy of palonosetron and ondansetron in prevention of postoperative nausea and vomitingInt Surg J 2015 2(1):549-55.10.18203/2349-2902.isj20151078 [Google Scholar] [CrossRef]
[24]. Moon YE, Joo J, Kim JE, Lee Y, Anti-emetic effect of ondansetron and palonosetron in thyroidectomy: A prospective, randomized, double-blind studyBr J Anaesth [Internet] 2012 108(3):417-22.Available from: http://dx.doi.org/10.1093/bja/aer42310.1093/bja/aer42322277663 [Google Scholar] [CrossRef] [PubMed]
[25]. De Leon A, Palonosetron (Aloxi): A second-generation 5-HT3 receptor antagonist for chemotherapy-induced nausea and vomitingProc (Bayl Univ Med Cent) [Internet] 2006 19(4):413-16.Available from: https://www.ncbi.nlm.nih.gov/pubmed/1710650610.1080/08998280.2006.1192821017106506 [Google Scholar] [CrossRef] [PubMed]
[26]. Naguib M, el Bakry AK, Khoshim MH, Channa AB, el Gammal M, el Gammal K, Prophylactic antiemetic therapy with ondansetron, tropisetron, granisetron and metoclopramide in patients undergoing laparoscopic cholecystectomy: A randomized, double-blind comparison with placeboCan J Anaesth 1996 43(3):226-31.10.1007/BF030117398829860 [Google Scholar] [CrossRef] [PubMed]