Expression of Brain Derived Growth Factor in Hippocampus of Mid Gestational Human Fetuses
Shilpi Garg1, Sabita Mishra2, Swati Tiwari3
1 Assistant Professor, Department of Anatomy, Faculty of Medicine and Health Sciences, SGT Medical College Hospital and Research Institute, Gurugram, Haryana, India.
2 Head and Director Professor, Department of Anatomy, Maulana Azad Medical College, New Delhi, India.
3 Assistant Professor, Department of Anatomy, Maulana Azad Medical College, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shilpi Garg, Assistant Professor, Department of Anatomy, Faculty of Medicine and Health Sciences, SGT Medical College Hospital and Research Institute, Gurugram, Haryana, India.
E-mail: mdshilpi@gmail.com
Introduction
Brain Derived Growth Factor (BDNF) is a sub member of neurotrophin family and is a critical regulator of formation and plasticity of neuronal networks in hippocampal formation. It acts in an activity dependent manner and its expression is highly sensitive to developmental and environmental factors.
Aim
To observe the expression of BDNF in the developing hippocampus of mid gestational aborted human fetuses.
Materials and Methods
In the present study 15 aborted fetuses from 14 to 30 weeks of gestation, were procured from the department of Obstetrics and Gynaecology, LN hospital after obtaining ethical clearance. For each gestational age the tissue was stained with cresylviolet and H&E to see the general morphology of hippocampus and immunostaining of the sections was done for the expression of BDNF.
Results
Subparts of hippocampus including Ammons horn, subiculum and dentate gyrus were identified in all age groups. Immunostaining was detected in both cell bodies and fibers. Expression of BDNF was more marked in the pyramidal cells of hippocampus and granule cell layer of the dentate gyrus of higher gestational age groups as compared to lower ones.
Conclusion
There is gradual increase in the BDNF expression as fetal age advances. Increased expression of BDNF in higher gestational age groups showed that neurotrophins like BDNF influence the neuronal differentiation and is important to neuronal survival.
Granule cells, Hippocampal plate, Intermediate zone, Marginal zone, Pyramidal cells, Ventricle zone
Introduction
The BDNF is a polypeptide growth factor. It activates high-affinity Tropomyosin receptor Kinase (Trk) as well as low affinity p75 neurotrophin receptor (p75NTR) [1,2]. Neurotrophins like BDNF direct growth and differentiation in the developing nervous system and helps in synaptogenesis, neuronal survival and promotes Long Term Potentiation (LTP) by modulating NMDA receptor subunit [3-6]. Its expression is highly sensitive to developmental and environmental factors. BDNF signaling enhances neurogenesis and electrophysiological activity reflective of general enhancement of hippocampal function [7]. Hippocampus is a part of limbic lobe emerging from the medial wall of temporal lobe and is associated with procuring of food, eating and emotional behaviour of the being. During development four fundamental embryonic zones i.e., Ventricle Zone (VZ), Intermediate Zone (IZ), Hippocampal Plate (HP) and Marginal Zone (MZ) has been identified as early as 9th week of gestation in hippocampus proper. Granule Cell Layer (GCL) is characteristic of dentategyrus [8-10].
This region has gained significance recently due to involvement of BDNF in development of hippocampus which is important for specific learning and memory process. Abnormal expression of BDNF is associated with various neuropathological disorders like schizophrenia, Alzheimer’s and depression [11-13]. The present study aimed to observe the expression of BDNF in the developing hippocampus of mid gestational human fetuses.
Materials and Methods
Fifteen aborted fetuses from 14-30 weeks of gestation were procured from the Department of Obstetrics and Gynecology, LN hospital after obtaining Ethical clearance (F.1/IEC/MAMC/(40)/6/2013/No:06) and written informed consent, in January 2014.
Gestational ages were determined by measuring various parameters, such as Crown Rump Length, Bi parietal Diameter and Foot Length and correlated with hospital data.
After procuring the fetus an incision was given on the scalp from the bregma along the sagittal suture for immediate fixation of the brain. The fetus was then immersion fixed in 4% paraformaldehyde. The brain was then removed from the cranial cavity after 24-72 hours and preserved in fresh fixative for 1-2 weeks. Brains that showed any degree of autolysis were not considered for the study. Slices of hippocampal area were dissected out. These were preserved in the fixative for 48 hours. Slices were labeled and processed for paraffin embedding. Seven micron thick serial sections were generated on a rotary microtome and every 3rd section was stained with Haematoxylin-Eosin stain. In each gestational age the 4th section and 5th section were processed for Nissl’s stain and BDNF, respectively. Sections after deparafinisation were treated with methanol and 1% H2O2 for blocking the endogenous peroxidase activity. After washing with distill water antigen retrieval (unmasking) was carried out by putting the slides for 7-8 minutes in a microwave for sections to be treated with primary antibody BDNF. Slides were completely immersed in the citrate buffer during this step. After washing with phosphate buffer slides were treated with normal horse serum for 2 hours for blocking non specific antigen. Following this, without washing, the sections were incubated with monoclonal antibody for BDNF at a dilution of 1:200. The reaction was visualised by biotinylated mouse secondary antibody and using DiaminoBenzidine as a chromagen. The sections were then examined under a BX61 motorised microscope and the images were captured with Olympus DP71 camera. Processing of images was done with ImagePro plus MC 6 software. Expression of BDNF was observed and analysed.
Results
The fetal measurements are shown in [Table/Fig-1] and the various fetal zones of hippocampus where the BDNF expressions were marked is shown in [Table/Fig-2].
Fetal measurements and collection data.
| Age (in weeks) | CRL (in cm) | BPD (in cm) | FL (in cm) | Number (Collected) | Gender (M/F) |
|---|
| 18 | 18-19 | 4.6-5.0 | 2.6-3.0 | 4 | 3-M |
| 1-F |
| 20 | 19-20 | 5.2-5.6 | 3.3-3.7 | 3 | 2-F |
| 1-M |
| 22 | 20-22 | 5.6-5.8 | 3.7-3.9 | 3 | 2-M |
| 1-F |
| 24 | 23-24 | 5.9-6.0 | 4.0-4.2 | 3 | 2-M |
| 1-F |
| 28 | 26-28 | 6.0-6.2 | 4.5-4.7 | 2 | 1-M |
| 1-F |
Expression of BDNF in various fetal zones of hippocampus in different gestational age groups.
| Fetal zone | | 18 wks | 20 wks | 22 wks | 24 wks | 28 wks |
|---|
| Ventricular Zone (VZ) | Neurons | Neuroblasts | Immature neuronal cells | Mature neuronal cells | More differentiated neuronal cells | Well differentiated neuronal cells |
| BDNF expression | + | + | ++ | +++ | +++ |
| Intermediate Zone (IZ) | Neurons | Immature neurons | Immature neurons | Mature neuronal cells | More differentiated neuronal cells | Well differentiated neuronal cells |
| BDNF expression | + | + | + | + | + |
| Hippocampal Plate (HP) | Neurons | Immature neurons | More differentiated neuronal cells | Few pyramidal cells | More differentiated pyramidal cells | Well differentiated pyramidal cells |
| BDNF expression | + | + | ++ | +++ | +++ |
| Dentate Gyrus (DG) | Neurons | Immature neurons | Immature neurons | Mature neuronal cells | More differentiated granular cells | Well differentiated granular cells |
| BDNF expression | + | + | ++ | +++ | +++ |
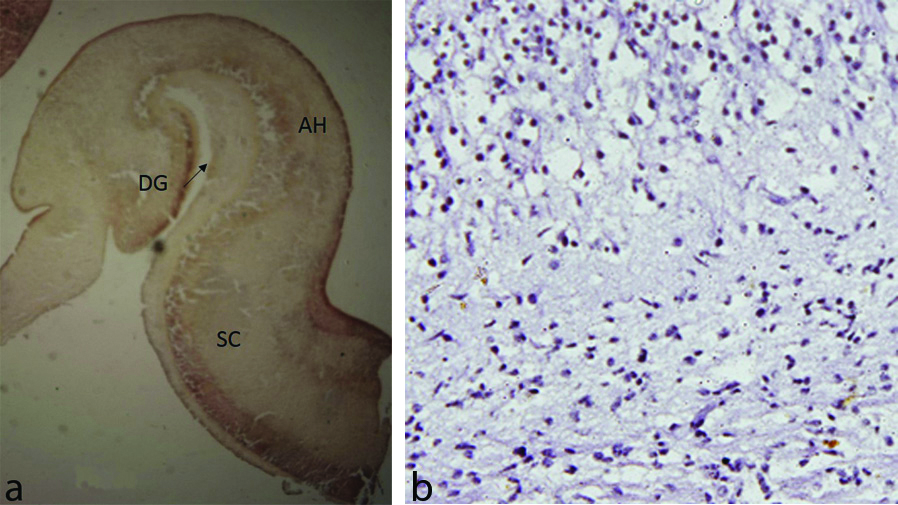
18th Gestational weeks
Hippocampus was clearly identified developing inside the cavity of inferior horn of lateral ventricle. The fetal zones VZ, IZ, HP and MZ were seen. BDNF expression was detected in both cell bodies and fibers and was more marked in VZ and GCL of dentate gyrus as compared to IZ and MZ [Table/Fig-3a,b].
a) Hippocampus showing Dentate Gyrus (DG), Ammon’s Horn (AH), Subicular Complex (SC) and Entorhinal Cortex (EC) around the hippocampal fissure (arrow) (18 week). b) Neuronal cells along with cell processes showing BDNF expression (18 week) (IHC stain, 40x).
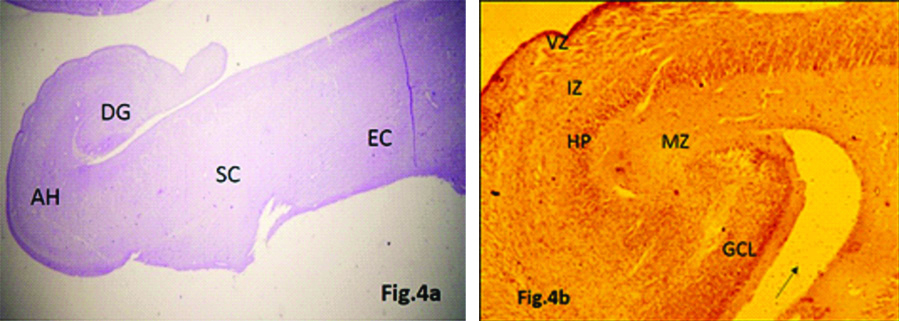
20th Gestational Weeks
The subparts of hippocampus (Ammon’s horn, subicular complex and dentate gyrus) were identified following the curve of hippocampal fissure. Fimbria was seen extending just above the dentate gyrus. BDNF expression was more marked in VZ, HP and granule cell layer of dentate gyrus (DG) as compared to IZ and MZ [Table/Fig-4a,b].
a) Hippocampus showing Dentate Gyrus (DG), Ammon’s Horn (AH), Subicular Complex (SC) and Entorhinal Cortex (EC) around the hippocampal fissure (arrow) (20 week) (H&E stain, 4x). b) BDNF expression seen in Ventricular Zone (VZ), Hippocampal Plate (HP), and Granule Cell Layer (GCL) of Dentate Gyrus (DG) around hippocampal fissure (arrow) (20 week) (IHC stain, 4x).
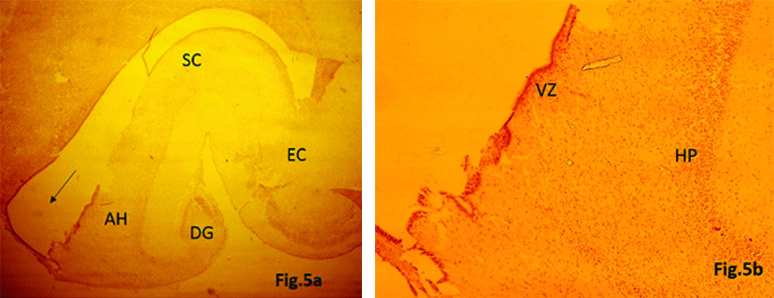
22nd Gestational Weeks
The hippocampus became more curved and mature in appearance. Subparts of hippocampus were clearly identified. BDNF expression was seen in all the fetal zones, though staining intensity was more marked in VZ and HP as compared to earlier gestational age groups [Table/Fig-5a,b].
a) Expression of BDNF in Hippocampus showing Dentate Gyrus (DG), Ammon’s Horn (AH), Subicular Complex (SC) and Entorhinal Cortex (EC) developing inside the inferior horn of lateral ventricular cavity (arrow) (22 week) (IHC stain, 4x). b) Neuronal cells along with cell processes showing BDNF expression in Ventricular Zone (VZ) and Hippocampal Plate (HP) (22 week) (IHC stain, 4x).
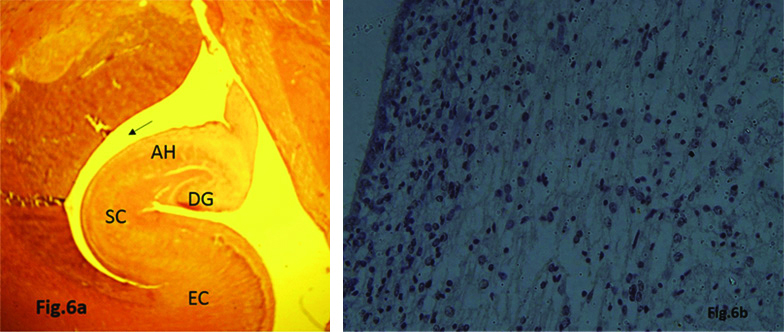
24th Gestational Weeks
Hippocampus attained more mature appearance developing inside the inferior horn of lateral ventricle cavity. Expression of BDNF was seen in both cell bodies and fibers and was more marked in HP and GCL of dentate gyrus as compared to earlier gestational age groups [Table/Fig-6a,b].
a) Expression of BDNF in Hippocampus showing Dentate Gyrus (DG), Ammon’s Horn (AH), Subicular Complex (SC) and Entorhinal Cortex (EC) inside the inferior horn of lateral ventricular cavity (arrow) (24 week). b) Neuronal cells along with cell processes showing BDNF expression (24 week)
28th Gestational Weeks
Hippocampus attained almost mature appearance. Subparts of hippocampus were clearly identified following the curve of hippocampal fissure. Hippocampal fissure had started to fuse with the main body of hippocampus. Maturation of cells was in more advanced stages. Expression of BDNF was seen more intense in cell bodies and fibers of VZ and Granule cell layer of DG as compared to earlier gestational age groups.
Expression of immunohistochemical marker (BDNF) is more marked (+++) in higher gestational age groups as compared to lower ones (+) in various fetal zones of hippocampus.
Discussion
The present study evaluated the expression of BDNF in the hippocampal formation of human fetuses ranging from 14th to 30th gestational weeks. This was a morphological study where we were able to observe the microscopic structure of hippocampal formation including dentate gyrus, cornuammonis and subicular complex and expression of BDNF was correlated. Neurotrophins like BDNF direct growth and differentiation in the developing nervous system. BDNF signaling enhances neurogenesis, neurite sprouting and electrophysiological activity [6,7].
The increase in BDNF expression in the temporal cortex of the neonates suggests that neurotrophin signaling is important in the early development of temporal cortex. BDNF and its receptors are expressed throughout the development and maturation of the human hippocampus [14].
BDNF regulates synaptic transmission and its release is activity dependent. BDNF is involved in trafficking NMDA receptor subunit to the plasma membrane of hippocampal neurons thereby increasing the potential for calcium influx. A sufficient local buildup of calcium causes postsynaptic BDNF release and promotes LTP, underlying mechanism for the retention of memory and learning process [15].
In the present study the lowest age of fetus included is 18 weeks where the primordial hippocampus was identified developing on the medial edge of inferior horn of lateral ventricle. Subparts of hippocampus were identified along the curve of hippocampal fissure in all the fetuses included in our study and four fundamental embryonic zones i.e., VZ, IZ, HP and MZ has been delineated. Expression of BDNF was detected in cell bodies and fibers of all age groups. BDNF expression was more marked in VZ, HP and GCL of dentate gyrus as compared to IZ and MZ. BDNF expression was more marked in the higher gestational age groups as compared to lower ones [Table/Fig-2]. Our findings corroborates with other studies [16-18] done on hippocampus of various animals for the expression of BDNF. BDNF mRNA is found to be lowest in the brains of early rats and increases into adulthood [16]. BDNF protein concentration increases over time during postnatal development of brain in albino rats and stabilises thereafter [17]. Infusion of BDNF into the adult brain in rats promotes neurogenesis and dendritic spine reorganisation in the rat hippocampal formation [18]. Increased expression of BDNF is seen in pyramidal layer of CA2 and CA3 in higher gestational age groups as compared to lower ones [19]. Decrease in expression of BDNF occurs in hippocampus during aging and in Alzheimer’s disease [12,13]. During brain development, the BDNF is involved in various processes like neurogenesis, gliogenesis, synaptogenesis, regulation of cell death and elimination of improperly formed connections [20]. The concentration of BDNF pro-domain rises during adolescence and adulthood, following the increase of m-BDNF. It is released from the neurons after depolarisation with definitive physiological properties [21]. BDNF is highly expressed in limbic cortex and is a crucial factor in regulation of learning and memory [22]. Impaired regulation of BDNF expression is involved in various cognitive and neurodegenerative diseases like mood and anxiety disorders, Alzheimer’s and Parkinson’s disease [23,24].
Limitation(s)
It was an observational study where the expression of BDNF was observedvia immuno histochemical procedure. For better quantitative analysis stereological methods should be considered.
Conclusion(s)
Though extensive studies has been done on lower animals, human studies are limited. Thus, from the present study done on human hippocampus it is evident that there is a gradual increase in the BDNF expression as the fetal age increases.
Declaration: This study was presented in the 63rd NATCON, National Conference of Anatomical Society of India at King George’s Medical University, Lucknow, India in 2015 as oral presentation.
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: May 17, 2016
Manual Googling: Feb 13, 2020
iThenticate Software: Feb 26, 2020 (11%)
[1]. Binder DK, Scharfman HE, Brain-derived neurotrophic factorGrowth Factors 2004 22(3):123-31.10.1080/0897719041000172330815518235 [Google Scholar] [CrossRef] [PubMed]
[2]. Götz R, Koster R, Winkler C, Raulf F, Lottspeich F, Schartl M, Neurotrophin-6 is a new member of the nerve growth factor familyNature 1994 6503(372):266-69.10.1038/372266a07969471 [Google Scholar] [CrossRef] [PubMed]
[3]. Pencea V, Bingaman KD, Wiegand SJ, Luskin MB, Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamusJ Neurosci 2001 17(21):6706-17.10.1523/JNEUROSCI.21-17-06706.2001PMC6763082 [Google Scholar] [CrossRef] [PubMed]
[4]. Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S, Increased neurogenesis and the ectopic granule cells after intra hippocampal BDNF infusion in adult ratsExp Neurol 2005 192(2):348-56.10.1016/j.expneurol.2004.11.01615755552 [Google Scholar] [CrossRef] [PubMed]
[5]. Xu B, Gottschalk W, Chow A, Louis F, Wilson R, Schnell E, The role of brain- derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving trk BJ Neurosci 2000 20(18):6888-97.10.1523/JNEUROSCI.20-18-06888.200010995833 [Google Scholar] [CrossRef] [PubMed]
[6]. Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB, BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neuronsMol Cell Neurosci 2007 35(2):208-19.10.1016/j.mcn.2007.02.01917428676 [Google Scholar] [CrossRef] [PubMed]
[7]. McAllister A, Katz L, Lo D, Neurotrophins and synaptic plasticityAnnual Review of Neuroscience 1999 22:295-318.10.1146/annurev.neuro.22.1.29510202541 [Google Scholar] [CrossRef] [PubMed]
[8]. Standring S, Gray’s Anatomy. The Anatomical Basis of Clinical Practice 2016 41st edNew YorkElsevier Churchill Livingstone:385-390. [Google Scholar]
[9]. Miller DB, O’Callaghan JP, Ageing, stress and the hippocampusAgeing Res Rev 2005 4(2):123-40.10.1016/j.arr.2005.03.00215964248 [Google Scholar] [CrossRef] [PubMed]
[10]. Garg S, Kaul JM, Mishra S, Vasudeva N, Morphological maturation of the hippocampus during 2nd and 3rd trimester in human fetuses. An immunocytochemistry studyJ Anat Soc India 2014 63(2):166-71.10.1016/j.jasi.2014.11.009 [Google Scholar] [CrossRef]
[11]. Hayashi M, Yamashita A, Shimizu K, Somatostatin and brain derived neurotrophic factor mRNA expression in the primate brain: decreased level of mRNAs during agingBrain Res 1999 749:283-89.10.1016/S0006-8993(96)01317-0 [Google Scholar] [CrossRef]
[12]. Murray KD, Gall CM, Jones EG, Isackson PJ, Differential regulation of brain derived neurotrophic factor and type II calcium/calmodulin- dependent protein kinase messenger RNA expression in Alzheimer’s diseaseNeuroscience 1994 60:37-48.10.1016/0306-4522(94)90202-X [Google Scholar] [CrossRef]
[13]. Phillips HS, Hains JM, Armanini M, Laramee GR, Jhonson SA, Winslow JW, BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s diseaseNeuron 1991 7:695-702.10.1016/0896-6273(91)90273-3 [Google Scholar] [CrossRef]
[14]. Webster MJ, Herman MM, BDNF and trk B mRNA expression in the hippocampus and temporal cortex during the human lifespanGene Expr Patterns 2006 6:941-51.10.1016/j.modgep.2006.03.00916713371 [Google Scholar] [CrossRef] [PubMed]
[15]. Patapoutian A, Reichardt LF, Trk receptors: Mediators of neurotrophin actionCurr Opin Neurobiol 2001 11(3):272-80.10.1016/S0959-4388(00)00208-7 [Google Scholar] [CrossRef]
[16]. Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, NT- 3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expressionNeuron 1990 5(4):501-09.0.1016/0896-6273(90)90089-X [Google Scholar] [CrossRef]
[17]. Katoh-Semba R, Takeuchi IK, Semba R, Kato K, Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brainJ Neurochem 1997 69(1):34-42.10.1046/j.1471-4159.1997.69010034.x9202291 [Google Scholar] [CrossRef] [PubMed]
[18]. Danzer SC, Crooks KRC, Lo DC, McNamara JO, Increased expression of brain-derived neurotrophic factor induces formation of basal dendrites and axonal branching in dentate granule cells in hippocampal explant culturesJ Neurosci 2002 22(3):9754-63.10.1523/JNEUROSCI.22-22-09754.200212427830 [Google Scholar] [CrossRef] [PubMed]
[19]. Kim JK, Jeon SM, Lee KM, Park ES, Cho HJ, Expression of brain-derived neurotrophic factor in the rat forebrain and upper brain stem during postnatal development: an immune histochemical studyNeuroscience 2007 146(3):1128-36.10.1016/j.neuroscience.2007.02.01717395388 [Google Scholar] [CrossRef] [PubMed]
[20]. Kowianski P, Lietazau G, Czuba E, Waskow M, Stelgia A, Morys J, BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticityCell Mol Neurobiol 2018 38:579-93.10.1007/s10571-017-0510-428623429 [Google Scholar] [CrossRef] [PubMed]
[21]. Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Lonescu MS, Deogracius R, BDNF and its propeptides are stored in presynaptic dense core vesicles in brain neuronsJ Cell Biol 2012 196(6):775-88.10.1083/jcb.20120103822412021 [Google Scholar] [CrossRef] [PubMed]
[22]. Boulle F, Van den Hove DL, Jakob SB, Rutten BP, Hamon M, Van OS, Epigenetic regulation of the BDNF gene: Implications for psychiatric disordersMol Psychiatry 2012 17:584-96.10.1038/mp.2011.10721894152 [Google Scholar] [CrossRef] [PubMed]
[23]. Murinova J, Hlavacova N, Chmelova M, Riecansky I, The evidence for altered BDNF expression in the brains of rats reared or housed in social isolation: A systematic reviewFront Behav Neurosci 2017 11:10110.3389/fnbeh.2017.0010128620285 [Google Scholar] [CrossRef] [PubMed]
[24]. Sampaio TB, Savall AS, Gutierrez MEZ, Pinton S, Neurotrophic factors in Alzheimer’s and Parkinson’s diseases: Implications for pathogenesis and therapyNeural Regen Res 2017 12(4):549-57.10.4103/1673-5374.20508428553325 [Google Scholar] [CrossRef] [PubMed]