International Federation of Gynaecology and Obstetrics (FIGO) incorporated peritoneal washing cytology as part of the staging procedure for ovarian carcinomas in 1975 [1] based on the traditional studies documenting that peritoneal washing cytology could detect a subclinical intraperitoneal extension effectively [2,3]. This could be a highly sensitive indicator of subclinical peritoneal dissemination even in interval debulking surgery occasionally performed for ovarian cancer treatment [4,5]. Particularly, patients with Stage I disease are those in whom the presence or absence of tumour cells have a significant influence on prognosis and the basic necessity of chemotherapy. Adjuvant platinum-based chemotherapy is given to most patients with Stage IC disease, although there has been debate about the absolute survival benefit in women with Stage IA and IB cancers who have had thorough surgical staging [6].
Latest staging guideline describes that retrieval of any peritoneal fluid or ascites is required and that, when there is none, washings of the peritoneal cavity should be performed [7], whereas this doesn’t mention the detailed methodological technique for it. In conventional CS, accurate identification of malignant, reactive mesothelial or inflammatory cells has occasionally posed a diagnostic problem since long before [8]. Studies investigating the role, utility and accuracy of CB method in the diagnosis of malignant ascites concluded that ovarian carcinoma was the most frequently detected primary cancer [9]. Malignant cells not present on CS usually could not be detected on CB either; though, there are some studies indicating that cases with undetermined malignancies on CS showed conclusive diagnosis with the aid of CB preparation [10]. The lack of consensus between the cytologic and histologic findings has been considered to be due to samplling error. In this regard, previous studies reported additional yield for the malignancy by CB compared with that obtained by CS alone [11,12].
One limitation of these studies is that they included such a variety of disease including benign tumour or non-tumour-oriented disease by different CS and CB techniques. From the gynaecologic point of view, it is important to know the benefits of CB exclusively on ovarian carcinoma, particularly histology-associated yield of ascites fluid smear. In order to clarify this irrational default concept, The present authors have attempted to analyse the ovarian cancer ascites from different perspective.
The long-term aim of this study are: (a) to compare the accuracy of either CS or CB or combination of each in relation to histologic type; (b) to determine whether the histology relevant false negative cytology occurs; and (c) to assess the available effect of CB technique.
Materials and Methods
This retrospective study was conducted in Jikei Daisan Hospital from January 2019 to August 2019. The study was performed with clinical and histopathological data from the hospital record. All ovarian cancer specimens for fluid cytology that consisted of both CS and CB over a 4-year period between 2014 and 2018 were reviewed and analysed. The study was approved by the Institutional Ethics Committee (30-330 (9351)) and Clinical Trial Review Board at The Jikei University School of Medicine. This study was carried out with explanations provided to the patients and a website with additional information, including an opt-out option for the study. The study conforms to the recognised standard of Declaration of Helsinki.
The exclusion criteria for the study are: (a) patients with a current diagnosis of borderline carcinomas; (b) patients with other malignancies including synchronous primary endometrial cancer or a past history of primary endometrial cancer; and (c) patients who have received prior chemotherapy or radiation therapy.
Out of 103 ovarian cancers the present authors have treated, 64 cases met the criteria and the histological diagnosis of primary tumour contained high grade serous, 27; clear cell, 17; endometrioid, 11; others including mucinous, 2; carcinosarcoma, 2; adenocarcinoma, 5. For the sample size calculation, it was assumed that the detection rate would be 0.60 in CS alone group and 0.75 in CB-combining groups with a significance level of one-sided 0.10 and a low statistical power of 0.70. This comes down to the estimated required sample sizes of 76 that were still more than the present examined number of cases. All ascites cytological specimens reviewed in this study were collected at the time of surgery. Any peritoneal fluid or ascites is retrieved and when there is none, washings of the peritoneal cavity have been performed as shown in guideline [7].
The fluid specimen was treated as quickly as possible after collection. Suboptimal preserved fluid specimens, Clotted fluid specimen and specimens of time between collection and processing more than one hour were excluded from the present study. Out of 50 mL of each fresh fluid specimen collected, 20 mL was subjected to the CS and 30 mL was for the CB technique.
Smearing technique: 20 mL of fluid sample was centrifuged at 3,000 rpm for three minutes, and centrifuged smears were prepared from deposits. At least 6 smears were prepared from the deposits. One smear was prepared after air drying and was stained with the May-Grünwald-Giemsa stain. The other 5 smears were immediately fixed in 95% alcohol and were stained with the Papanicolaou stain. Additional Haematoxylin-Eosin, Alcian Blue and PAS staining was carried out when necessary. All smears were prepared using carbowax-coated slides. Cytologic diagnosis reported before histologic diagnosis is completed.
Cellblocking: Cellblock procedure is based on Japanese guideline for cytology [13]. 30 mL of entire material was centrifuged in an Eppendorf tube at 3,000 rpm for 3 minutes to create >0.5 mL cell pellets. The supernatant fluid was decanted and the deposit fixed in 20% formaldehyde for 24 hours. The cellblocks were embedded in paraffin and sectioned at 3 μm thickness. Routine H&E staining was used on all cellblock sections and, when necessary, immunohistochemistry was performed including cytokeratin-7, -20, calretinin, PAX8, WT1, D2-40 and CAM5.2. Cellblock diagnosis is also reported independently from histologic diagnosis of the tumour.
All smears and corresponding CB were reviewed separately. CS was categorised into three groups based on the final cytological diagnosis: benign (Class I or II), atypical cells (class III), and malignant (Class V). For this study, the last group was designated as positive for cytology, whereas the CB was grouped simply in two categories; benign or malignant. In this study, sensitivity for CS and CB is designated as follows;
Sensitivity for CS=number of CS positive case/total number of either or both of CS or CB positive case
Sensitivity for CB=number of CB positive case/total number of either or both of CS or CB positive case
Statistical Analysis
Differences between samples or groups of samples were determined by likelihood-ratio of Pearson’s chi-square test with critical level of significance fixed at one-sided 0.05. For the sample size analysis, estimated sample size for two-sample comparison of proportions was calculated. The statistical analysis was conducted using STATA (Stata/MP 12.1) College Station, TX 77845, USA.
Results
Sensitivity and yield for malignancy of conventional smears and cellblocks. Out of 103 ovarian cancer cases, 64 peritoneal fluid samples were subjected to CS and CB. Sensitivity information by histologic type is given in [Table/Fig-1]. The overall sensitivity of CS (91%) was almost the same as CB (95%) and the same trend was noted on high grade serous (100% vs 100%; N=27) and clear cell carcinoma (100% vs 92%; N=17). The yield for malignancy in overall analysis could be improved slightly by 6.3% (4/64) when CB was utilised together with CS, while no improvement on definitive cytologic conclusion for malignancy was observed on high grade serous nor on clear cell carcinoma. Whereas, in the endometrioid carcinoma (N=11), the sensitivity of CS (20 %) was remarkably inferior to CB (80%). The yield for malignancy on endometrioid carcinoma was improved by 36.3% (4/11) when CB was combined with CS. The likelihood-ratio of Pearson’s chi-square was 3.922 with the p-value of 0.048 and the result was statistically significant. On the other hand, two cases showed positive CS with negative CB (one of each clear cell and endometrioid carcinoma). Those false-negative CB were caused by inadequate CB presentation, low cellularity and/or poor sample processing.
Comparison of positivity on cellblock with conventional smear by histology.
| All cases | High grade serous | Clear cell | Endometrioid |
|---|
| CS + | CS - | Total | CS + | CS - | Total | CS + | CS - | Total | CS + | CS - | Total |
|---|
| CB + | 37 | 4 | 41 | 20 | 0 | 20 | 11 | 0 | 11 | 0 | 4 | 4 |
| CB - | 2 | 21 | 23 | 0 | 7 | 7 | 1 | 5 | 6 | 1 | 6 | 7 |
| Total | 39 | 25 | 64 | 20 | 7 | 27 | 12 | 5 | 17 | 1 | 10 | 11 |
| Sensitivity* |
| Smear | 0.907 | 1.000 | 1.000 | 0.200 |
| Cell block | 0.953 | 1.000 | 0.917 | 0.800 |
CS: Conventional smear; CB: Cellblock; *sensitivity for CS=number of CS positive case/total number of either or both of CS or CB positive case; sensitivity for CB=number of CB positive case/ total number of either or both of CS or CB positive case
Presentation of the four cases exhibiting negative CS and positive CB [Table/Fig-2] summarised the clinical features of those selected four cases exhibiting negative CS with positive CB. Except for case two, all patients received curative surgery. Confirmed with positive CB, patients were administered regular adjuvant chemotherapy. For the sample size calculation exclusively in endometrioid carcinoma, it was assumed that the yield for malignancy could be 0.10 in CS alone group and 0.50 in CB-combining group with a significance level of one-sided 0.10 and a lower statistical power of 0.70. This resulted in the estimated required sample sizes of 13, indicating that two more cases were needed for verification of data even with lower statistical power.
Clinical features of patients.
| Case | Age (years) | Para | Menopause (years old) | Surgery | Comorbidity of endometriosis | Ascites | Chemo-Tx |
|---|
| 1 | 65 | G0P0 | 52 | Hx, BSO, OMX, LNX | (+) | (+) | TC |
| 2 | 30 | G0P0 | not yet | RSO, OMX | (+) | (+) | Unknown* |
| 3 | 67 | G0P0 | 50 | Hx, BSO, OMX, LNX | (+) | (+) | TC |
| 4 | 57 | G0P0 | 55 | Hx, BSO, OMX, LNX | (+) | (+) | TC |
Hx: Hysterectomy; RSO: Right salpingo-oophorectomy; BSO: Bilateral salpingo-oophorectomy; OMX: Omentectomy, LNX: Lymphadenectomy; Chemo-Tx.: Chemotherapy; TC: paclitaxel-carboplatin; *Unknown; Patient moved to another hospital
The [Table/Fig-3] listed histological findings of each case. All patients had endometrioid carcinoma. The [Table/Fig-3] also shows the revision of staging by positive CB diagnosis in case 1, 2 and 3, resulting in the indication of adjuvant chemotherapy.
Histological findings and clinical staging.
| Case | Ascites cytology | Cellblock | Histology | Stage (FIGO) | Revised stage* (FIGO) |
|---|
| 1 | Class II Reactive mesothelial cells | Adenocarcinoma suspicious of ovarian cancer | Endometrioid carcinoma, Grade 3 | pT1aN0M0 (IA) | pT1c3N0M0 (IC3) |
| 2 | Class II Mesothelial cells | Malignant cells suspicious of adenocarcinoma | Endometrioid carcinoma, Grade 1 | pT1cNXM0 (IC1) | pT1cNXM0 (IC3) |
| 3 | Class III Mesothelial cells | Malignant cells compatible with endometrioid carcinoma | Endometrioid carcinoma, Grade 2 | pT1aN0M0 (IA) | pT1cN0M0 (IC3) |
| 4 | Class III Reactive mesothelial cells | Malignant cells | Endometrioid carcinoma, Grade 1 | pT2N1M0 (IIIA1) | pT2N1M0 (IIIA1) |
*Staging revised by cellblock
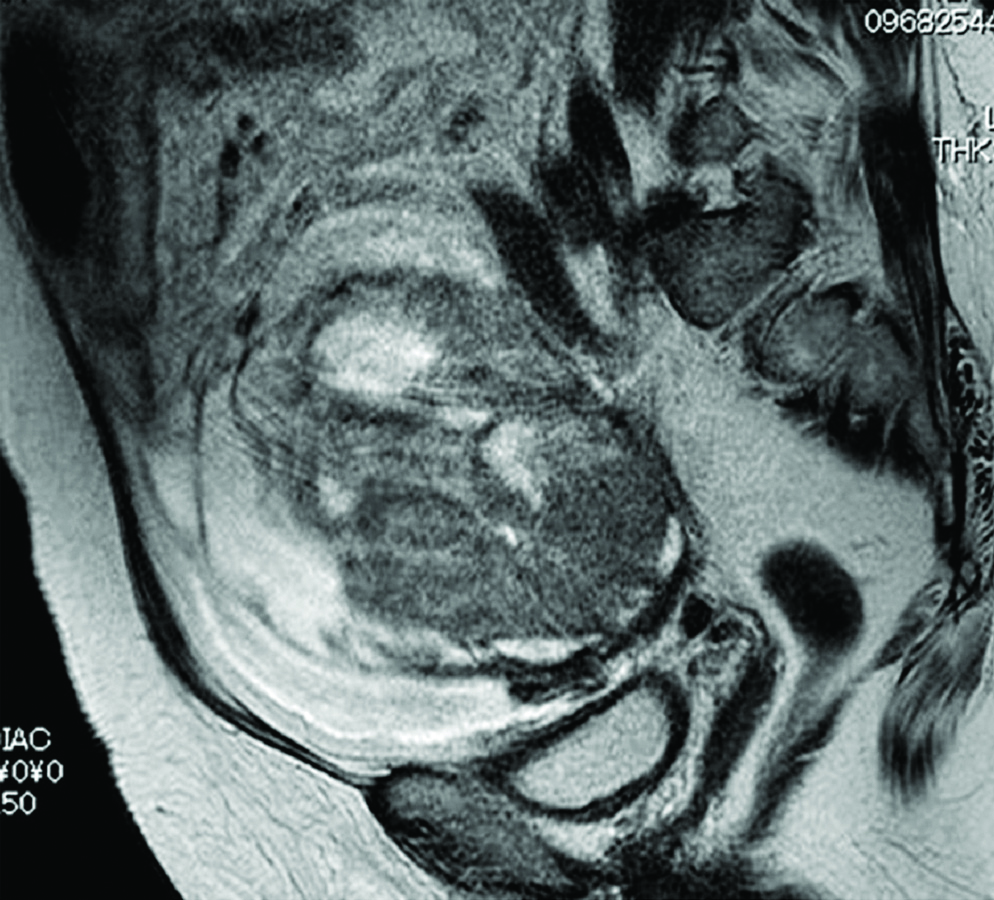
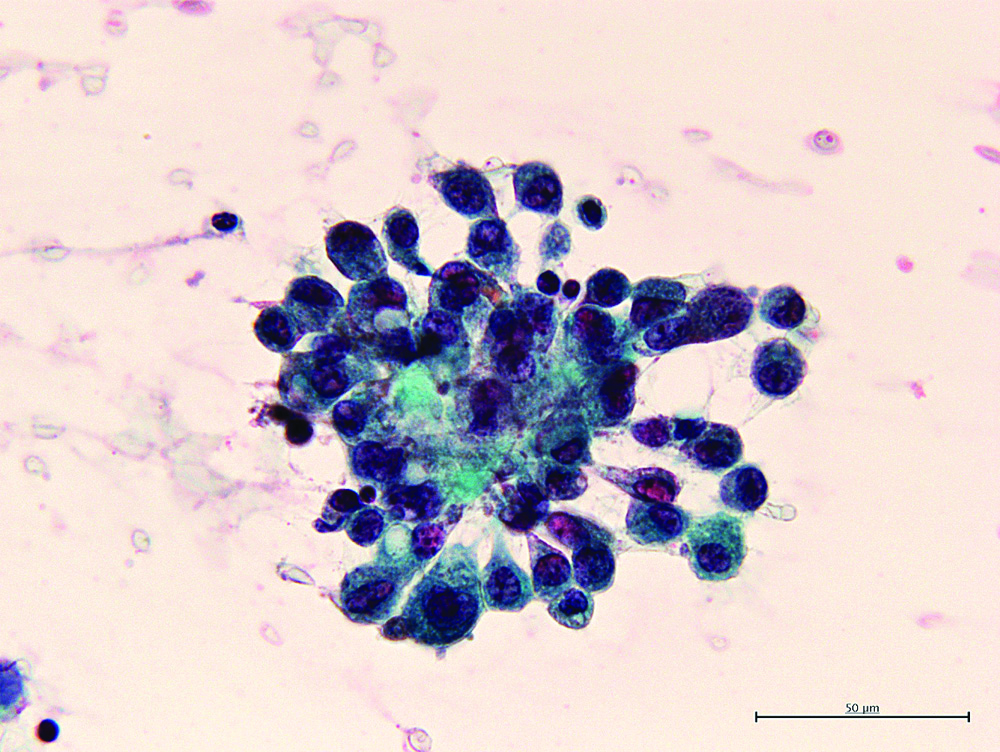
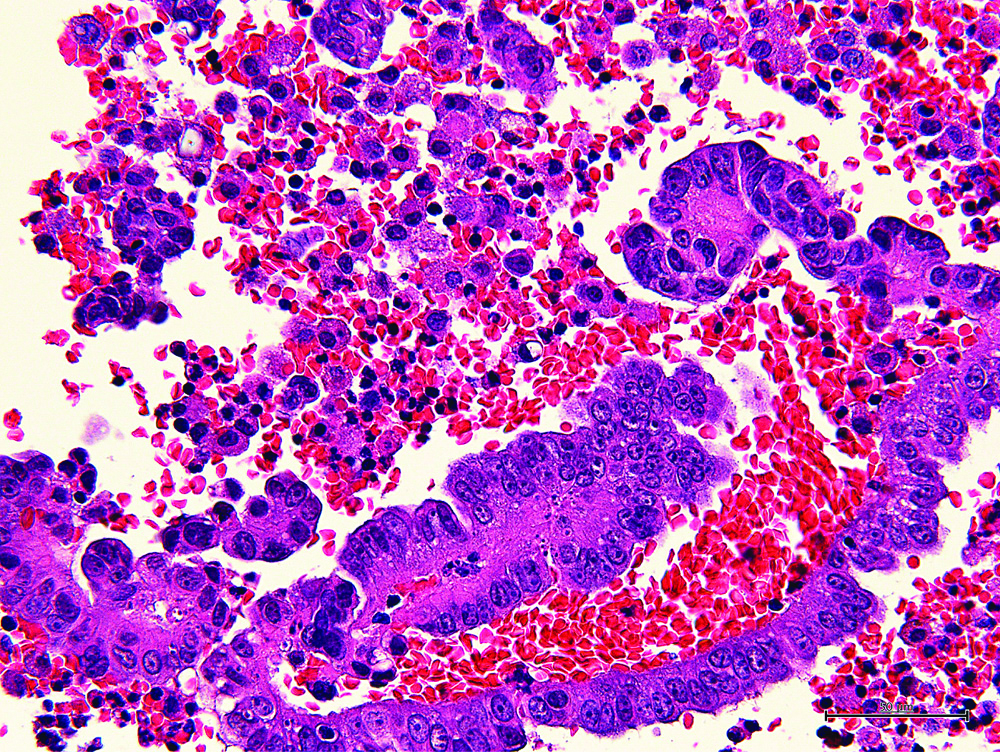
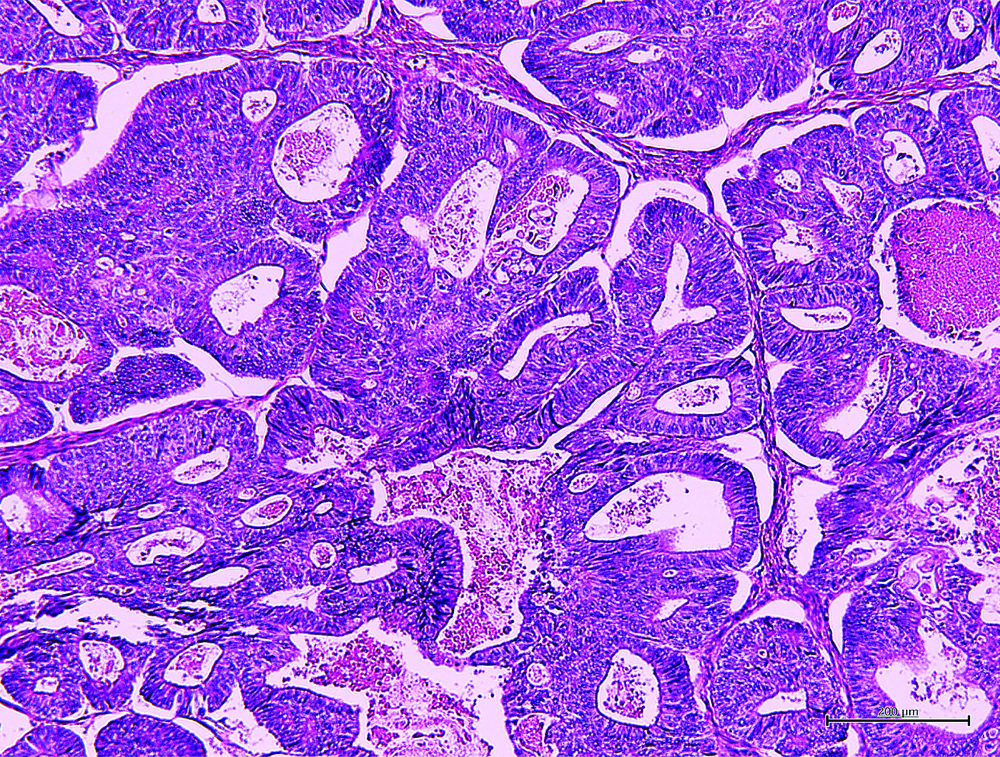
The [Table/Fig-4] shows the representative MR image of case III presenting a huge cystic and solid blended mass extending 130 mm with ascites suggesting diagnosis of ovarian carcinoma. The [Table/Fig-5] shows representative cytologic evidence of reactive mesothelium on case III. Impression smears have predominantly neutrophilic inflammation and clusters of reactive mesothelial cells with a moderately enlarged nucleus, coarsely stippled chromatin, small to moderately sized, variably distinct nucleoli and basophilic cytoplasm occasionally containing mucinous material, being compatible with class III. The [Table/Fig-6] shows representative CB evidence of case III. Among erythrocytes, lymphocytes, histiocytes, neutrophils, and reactive mesothelial cells, clusters of columnar cells with eosinophilic cytoplasm and large oval nuclei containing clear nucleoli are sporadically observed. Occasionally, the cells form small glandular structures. These findings are consistent with endometrioid carcinoma. The [Table/Fig-7] shows representative pathology of case III. The glands are small and round, and they are lined by columnar cells with large atypical oval nuclei and basophilic cytoplasm. The cells grow in single or multiple layers. These findings are compatible with endometrioid carcinoma.
Representative MR Image of case III. A huge cystic and solid blended mass extending 130 mm with large amount of ascites.
Representative cytologic evidence of reactive mesothelium on case III. Clusters of reactive mesothelial cells with a moderately enlarged nucleus, coarsely stippled chromatin, variably distinct nucleoli and basophilic cytoplasm could be noted 600X.
Representative cellblock evidence of case III. Clusters of columnar cells with large oval nuclei containing clear nucleoli are observed. The finding is consistent with endometrioid carcinoma 400X.
Representative pathology of case III. Typical endometrioid carcinoma was clearly shown 100X.
Discussion
The present study presented additional CB technique could produce better yield by 6.3% (4/64) compared with CS alone and this effect was statistically significant when the cases were confined exclusively to endometrioid carcinoma (36.3%; 4/11) even though the lower sample size. In those selected four cases exhibiting negative CS with positive CB, the staging diagnosis was up-graded (three cases) and the chemotherapy was performed by that diagnosis (one case). Also, those cases exhibited the proposed risk factors common to the four cases [Table/Fig-2,3] including: (i) comorbidity of endometriosis; and (ii) histological type of endometrioid carcinoma.
Previous report Nathan NA et al., showed that the sensitivity of Papanicolaou-stained smear was slightly superior to cellblock though, in these days, few studies have compared the value of cellblock with smear [14]. In the area of head and neck pathology on fine needle aspiration, smear showed 42 % similarity with that of tissue biopsy, while cellblock showed 100% coincident with tumour biopsy [15]. Likewise, in case of pleural effusion, sensitivity was 81% on liquid based cytology compared with 94% on cellblock [16]. Both of these recent reports documented the diagnostic advantage for cellblock over smear. Further, compared with smear alone, the additional yield for the malignancy by combining cellblock with conventional smear was reported [11,12].
The present study centred exclusively on epithelial ovarian carcinoma where the cytologic diagnosis of ascites is weighed on diagnostic staging much more than other disease documented in above reports. The study also focused on pathology-oriented inconsistency on the sensitivity as well as the yield of CS and CB, as histologic type is the primary important factor for diagnosis of ovarian carcinoma. Considering those perspectives, the present authors have discussed by focusing on the following two points regarding to false negative smears: (a) classical possibility of sampling errors; and (b) histology associated incidence of false negative cytology.
First, authors argued the cause of false-negative cytology from technical perspective. Sampling errors have been long considered to occur in unsatisfactory collected cells [17], or low cellularity [18]. During collection or processing, the tumour cells may degenerate and become non-diagnostic. However, in this study CS [Table/Fig-5] exhibited satisfactory number of cells stained in high quality, resulting in the same degree of overall sensitivity of CS as CB. On the contrary, false-negative on CB in the present study could be attributable to sampling errors as our two cases presenting negative CB with positive CS [Table/Fig-1] exhibited extreme low cellularity on CB.
Second, authors contemplated the cause of false-negative cytology that was detected exclusively in cases with the two aforementioned features and focused on the possibility of stepwise malignant transformation on coexisting endometriosis. Atypical endometriosis is more frequently found in endometriosis with malignant tumours than in benign endometriotic cysts [19,20], and associates frequently with a continuous transition from benign endometriosis to carcinoma. Process of development into atypia and cancer in endometriosis is still not clear though, carcinogenic stepwise processes from benign endometriosis to carcinoma might differentially occur based on each histologic type. In the present study, comorbidity of endometriosis is 11.1% (3/27) in high grade serous, 71% (12/17) in clear cell carcinoma, and 91% (10/11) in endometrioid carcinoma (data not shown), indicating that clear cell and endometrioid adenocarcinomas are predominant. Divergent carcinogenic process could produce dissimilarities in cellular biological characteristics resulting in differential detection rate between CS and CB. Among atypical cells, genetically-encoded prospective clear cell carcinoma could display carcinomatous appearance on CS. Whereas those prospective endometrioid carcinoma cells could not exhibit carcinomatous appearance on CS while, when aggregated in CB, they construct the carcinomatous architecture like the case of endometriosis where CB shows the diagnostic advantage over CS [21]. Hence the malignant cells in ascites are hardly detected on CS in cases with endometrioid ovarian carcinoma with comorbidity of endometriosis.
Limitation(s)
In this kind of clinical study, the real number of genuine positivity on ascites cytology could not be properly clarified. The CS-false negative should be relied on CB and the CB-false negative should be relying on CS. Consequently, it is impossible to calculate the specificity. The study also has the severe limitation of sample size; the reviewed number of cases in the present study could not be amassed to the length of estimated sample size. Despite the specific statistical significance in endometrioid carcinoma, the sample size was not justified in this study.
Conclusion(s)
In this study, the finding presented that CB technique combined with CS provided significant improvement on the yield for malignancy compared to CS alone specifically to endometrioid carcinoma. As our take home message, authors would emphasise that CB along with CS should be considered particularly for early stage endometrioid ovarian carcinoma with comorbidity of endometriosis.
In future study, to collect the satisfying number of cases, multi-institutional, prospective clinical trial is further mandate.
CS: Conventional smear; CB: Cellblock; *sensitivity for CS=number of CS positive case/total number of either or both of CS or CB positive case; sensitivity for CB=number of CB positive case/ total number of either or both of CS or CB positive case
Hx: Hysterectomy; RSO: Right salpingo-oophorectomy; BSO: Bilateral salpingo-oophorectomy; OMX: Omentectomy, LNX: Lymphadenectomy; Chemo-Tx.: Chemotherapy; TC: paclitaxel-carboplatin; *Unknown; Patient moved to another hospital
*Staging revised by cellblock