IE is a microbial infection of heart valve or mural endocardium. The incidence of the disease has been estimated to be 3-10 per 1,00,000 persons per year by studies done in western countries [1,2]. Developing countries lack such data on incidence of IE as there is a paucity of reports. There has been an upward shift in the mean age of the patients with IE over the past decades owing to the improved living standards and advancements in healthcare facilities [3]. Diagnosis of the disease is done using modified Duke’s criteria which is dependent on blood culture and echocardiography. Echocardiography is a useful diagnostic modality but it has to be interpreted correctly in consideration with other clinical features required for making a definitive diagnosis. High income countries have a higher percentage of blood culture positivity (60-80%) when compared to low income and developing countries (40-60%) [4-9]. This may be due to antibiotic therapy prior to sample collection or infections caused by fastidious organisms. High incidence of Blood Culture Negative Endocarditis (BCNE) thus poses a problem in the management of the disease, and additional diagnostic criteria would be useful for early diagnosis. Inflammation forms an essential component in the pathogenesis of IE. Measuring the levels of inflammatory mediators can thus contribute to the rapid diagnosis of this condition [10,11]. Cytokines are inflammatory mediators which are produced when sensitised lymphocytes come in direct contact with an antigen. Quantifying cytokine levels in IE patients and comparing it with that of patients with non-IE infections and healthy controls will enable us to identify the panel of cytokines which may be useful as a new supportive diagnostic criterion especially in BCNE and atypical IE cases [12,13].
Hence, the present study aims at determining the levels of various cytokines in serum of IE patients in comparison with Pyrexia of Unknown Origin (PUO) patients and healthy individuals in order to understand the usefulness of cytokine assays, in the diagnosis of this disease.
Materials and Methods
Patient Population and Ethical Considerations
The analytical study was done using non-probability consecutive sampling method and was conducted in Dr. ALM PG Institute of Basic Medical Sciences, University of Madras, Chennai, Tamil Nadu, India. The study was approved by the Institutional Ethics Committee (IHEC NO: UM/IHEC/F.RM/2019-X). Informed and written consent was obtained from all participants.
Inclusion Criteria: Fifty two patients who presented to the Cardiology Department of Rajiv Gandhi Government General Hospital, Chennai between February 2017 and June 2018, diagnosed with IE as per the Modified Duke’s criteria [14] and who gave consent.
Exclusion Criteria: Patients with other valvular heart diseases without any infective aetiology were excluded.
IE cases underwent careful clinical examination and were subjected to laboratory investigations like blood culture, complete blood count, serum chemistry and urine analysis. Clinical history and echocardiography details were recorded. Two control groups (10 subjects in each group) were included. The first group comprised of 10 patients admitted with PUO for infections (Non-IE controls) other than endocarditis as evaluated with Duke Criteria and were selected from the same hospital. The second group comprised of 10 healthy individuals (Healthy controls) who did not show symptoms of any infection nor had any health related complaints and were selected from the community. Blood samples were obtained from all participants on admission for culture and serum separated for assessment of various cytokines levels. Blood culture was performed using standard procedures [15]. Two sets of blood samples were obtained from each patient with a time interval of one hour between each sample and inoculated in brain heart infusion broth with 0.04% sodium polyanethol sulfonate (HiMedia, Mumbai, India) and incubated at 37°C in a 5% CO2 atmosphere for 14 days and observed for turbidity every day.
Cytokine ELISA Assay
Serum samples from the patients with IE and from the controls were analysed for cytokines, IL-1β, IL-6, IL-8, IL-10, IL-12, TNF-α and IFN-γ using quantification ELISA [10,16] with Picokine ELISA 96 well kit (Boster biological technology, CA), according to the manufacturer’s instructions. Briefly, the samples were thawed and kit reagents were brought to room temperature. After appropriate dilution, 100 μL of standard, samples and controls were added to the respective wells precoated with murine monoclonal antibody and incubated for 120 minutes at room temperature, after which the contents of the well were discarded and 100 μL of 1x biotinylated anti-human antibody was added to each well and incubated for 90 minutes at room temperature. The plate was washed thrice and 100 μL of 1x Avidin-Biotin-Peroxidase complex was added to all wells and incubated for 40 minutes at room temperature. After washing the plate five times, 90 μL of substrate solution was added to each well and incubated in the dark for 30 minutes at room temperature, after which 100 μL of stop solution was added to each well and the plate was read at 450 nm. A calibration curve was constructed using mean absorbance obtained from each standard in duplicate. The cytokine concentration of each sample was then extrapolated from the standard curve.
Statistical Analysis
Categorical data were presented as numbers and percentages, continuous data were expressed as mean±Standard Deviation (SD). Serum cytokine concentrations between the groups were compared using Kruskal Wallis test. Statistical significance was assumed at p<0.05. Multiple comparison among study groups was done using post-hoc test sign test. ROC curve analysis was done to identify the cut-off level with optimum sensitivity and specificity of the marker/target cytokines. All analyses were performed using SPSS statistical software (version 21.0).
Results
Cases
Mean age of IE patients was 33.6 years (range-13-71 years). Among them, 34 (65.38%) were males and 18 (34.62%) were females. The most common underlying heart disease was rheumatic valvular disease (40.4%) [Table/Fig-1]. The mitral valve was the most frequently involved valve, which was affected in 57.7% of cases.
Characteristics of infective endocarditis patients (n=52).
| Predisposing factors | | Number (percentage) |
| Rheumatic disease | 21 (40.4%) |
| Intracardiac device | 2 (3.8%) |
| Prosthetic valve | 7 (13.5%) |
| Congenital heart disease | 7 (13.5%) |
| Prior infective endocarditis | 4 (7.7%) |
| Valve involved | | Number (percentage) |
| Mitral valve | 30 (57.7%) |
| Aortic valve | 14 (26.9%) |
| Tricuspid | 4 (7.7%) |
| Pulmonary valve | 1 (1.9%) |
| Mitral and aortic | 2 (3.8%) |
| Mitral, tricuspid and aortic | 1 (1.9%) |
| Associated conditions | | Number (percentage) |
| Diabetes mellitus | 11 (21.2%) |
| Chronic renal failure | 7 (13.5%) |
| Pharmacological immune suppression | 1 (1.9%) |
| Hepatic insufficiency | 2 (3.8%) |
| Aetiologic agents | | Number (percentage) |
| Staphylococcus aureus | 3 (5.8%) |
| Enterococci faecalis | 3 (5.8%) |
| Echocardiographic findings | | Number (percentage) |
| Chordae rupture | 5 (9.6%) |
| Intracardiac mass | 34 (65.4%) |
| Laboratorial data | | Mean±SD |
| Haemoglobin (g/dL) | 10.1±2.8 |
| White blood cell count (cells/mm3) | 12175.6±4157.2 |
The common clinical manifestations of the IE patients at the time of admission are mentioned in [Table/Fig-2]. Fever (65.4%) and dyspnoea (53.8%) were found to be the most common presenting symptoms in IE patients.
Common clinical manifestations of infective endocarditis patients (n=52).
| Clinical presentation | Number (percentage) |
|---|
| Fever | 34 (65.4%) |
| Dyspnoea | 28 (53.8%) |
| Malaise | 25 (48.1%) |
| Palpitations | 11 (21.2%) |
| Chest pain | 8 (15.4%) |
| Joint pain | 6 (11.5%) |
| Neurological complications | 8 (15.4%) |
Laboratorial Data
Out of 52 IE patients, blood culture was positive in six patients (11.5%). Staphylococcusaureus was isolated in 3 (50%) IE cases and Enterococcusfaecalis was isolated in the remaining 3 (50%). Culture-negative endocarditis occurred in 46 patients (88.5%). Anaemia was present in 71.2% (Haemoglobin <13.5 g/dL in men and <12 g/dL in women) of the IE patients, and White Blood Cell count (WBC) was elevated (WBC count >11,000 cells/mm3) in 59.6%. Echocardiography was performed in all patients; vegetations were found in 34 patients (65.4%).
Control Groups
Mean age of healthy controls was 34 (range-14-72 years). Among them seven were male and three were females. Among the non-IE Controls, six were males and four were females. The mean age of non-IE controls was 34.6 years (range 15-71 years).
Serum Concentrations of the Cytokines
The results of the serum concentrations of the cytokines (IL-1β, IL-6, IL-8, IL-10, IL-12, TNF-α and IFN-γ) of IE patients and controls are presented in [Table/Fig-3]. Except IL-10 and IL-12, all the other cytokine concentrations showed significant variation between the groups. The mean value of cytokine concentration was significantly higher among the cases when compared with the controls.
Descriptive statistics and comparison of serum cytokine concentrations.
| Serum cytokine concentrations (pg/mL) | Groups | Number | Minimum | Maximum | Mean | Standard deviation | Standard error | 95% CI LB§ | 95% CI UB§ | p-valueII |
|---|
| *IFN-γ | IE† | 52 | 131.00 | 227.00 | 155.84 | 30.76 | 4.27 | 147.28 | 164.41 | <0.001 |
| Non-IE‡ | 10 | 6.80 | 69.80 | 36.89 | 20.98 | 6.64 | 21.88 | 51.90 |
| Healthy | 10 | 5.40 | 69.90 | 47.67 | 24.68 | 7.80 | 30.02 | 65.33 |
| *TNF-α | IE† | 52 | 5.40 | 78.60 | 13.85 | 17.96 | 2.49 | 8.85 | 18.85 | <0.001 |
| Non-IE‡ | 10 | 6.30 | 12.50 | 7.58 | 1.99 | 0.63 | 6.16 | 9.01 |
| Healthy | 10 | 0.00 | 1.90 | 0.67 | 0.59 | 0.19 | 0.25 | 1.09 |
| *IL-1β | IE† | 52 | 27.00 | 1130.70 | 335.27 | 234.66 | 32.54 | 269.94 | 400.60 | 0.001 |
| Non-IE‡ | 10 | 7.20 | 495.10 | 243.00 | 187.12 | 59.17 | 109.14 | 376.85 |
| Healthy | 10 | 13.40 | 506.60 | 89.39 | 147.54 | 46.66 | -16.16 | 194.93 |
| *IL6 | IE† | 52 | 43.50 | 116.60 | 75.57 | 15.31 | 2.12 | 71.30 | 79.83 | <0.001 |
| Non-IE‡ | 10 | 41.10 | 81.30 | 59.18 | 14.66 | 4.64 | 48.69 | 69.67 |
| Healthy | 10 | 6.00 | 71.40 | 26.44 | 24.76 | 7.83 | 8.73 | 44.15 |
| *IL8 | IE† | 52 | 3.40 | 528.40 | 354.50 | 118.06 | 16.37 | 321.63 | 387.36 | <0.001 |
| Non-IE‡ | 10 | 7.90 | 472.90 | 141.22 | 144.85 | 45.81 | 37.60 | 244.83 |
| Healthy | 10 | 0.00 | 318.90 | 72.32 | 98.46 | 31.14 | 1.88 | 142.75 |
| *IL10 | IE† | 52 | 5.40 | 113.10 | 18.15 | 24.98 | 3.46 | 11.19 | 25.11 | 0.619 |
| Non-IE‡ | 10 | 5.80 | 57.80 | 18.00 | 18.22 | 5.76 | 4.97 | 31.03 |
| Healthy | 10 | 5.80 | 99.00 | 16.48 | 29.07 | 9.19 | -4.31 | 37.27 |
| *IL12 | IE† | 52 | 8.30 | 645.30 | 76.36 | 94.78 | 13.14 | 49.97 | 102.75 | 0.137 |
| Non-IE‡ | 10 | 2.90 | 134.40 | 62.62 | 49.09 | 15.53 | 27.50 | 97.74 |
| Healthy | 10 | 0.00 | 61.60 | 31.71 | 18.96 | 6.00 | 18.14 | 45.27 |
*: IFN-γ – Interferon gamma; TNF-α: Tumour necrosis factor alpha; IL-1β: Interleukin 1 beta; IL6: Interleukin 6; IL8: Interleukin 8; IL10: Interleukin 10; IL12: Interleukin 12; †-Infective endocarditis; ‡-Non-infective endocarditis; §-CI: Confidence interval; LB: Lower bound, UB: Upper bound; ||: Kruskal Wallis test for comparison of means
Multiple Comparisons among Study Groups
Multiple comparisons were done between the study groups using post hoc test sign test [Table/Fig-4]. The mean difference in TNF-α, IL-6 and IL-8 levels were found to be significant when IE cases were compared with healthy controls. Mean difference in IFN-γ levels were found to be significant when IE cases were compared with healthy controls as well as non-IE controls.
Post-hoc analysis of levels of cytokines among patients with infective endocarditis, non-infective endocarditis and healthy controls.
| Cytokine | IE cases versus Healthy controls p-value** | IE cases versus Non-IE controls p-value** |
|---|
| IFN-γ | 0.002 | 0.002 |
| TNF-α | 0.002 | 0.344 |
| IL-1β | 0.109 | 0.754 |
| IL-6 | 0.021 | 0.344 |
| IL-8 | 0.002 | 0.18 |
| IL-10 | 1 | 0.754 |
| IL-12 | 0.109 | 0.508 |
**post-hoc test sign test using binomial distribution
ROC Analysis
ROC analysis of the various cytokine levels showed a greater Area Under Curve (AUC) among IFN-γ, TNF-α, IL-1β, IL-6 and IL-8 levels when IE cases were compared with Healthy Controls (HC) [Table/Fig-5,6]. IL-6 and IL-8 at an optimum cut-off level of 56pg/mL and 204.39 pg/mL respectively, showed sensitivity 92.3%, specificity 90%, Positive Predictive Value (PPV) 97.96% (88.19%-99.68%) and Negative Predictive Value (NPV) 69.23% (46.18%-85.51%). (IL)-1β at a cut-off level of 100.02 pg/mL gave a sensitivity of 86.5%, specificity of 90%, PPV 97.83% (87.48%-99.66%) and NPV 56.25% (38.51-72.53%).
ROC for different serum cytokine concentrations comparing IE patients with healthy controls.
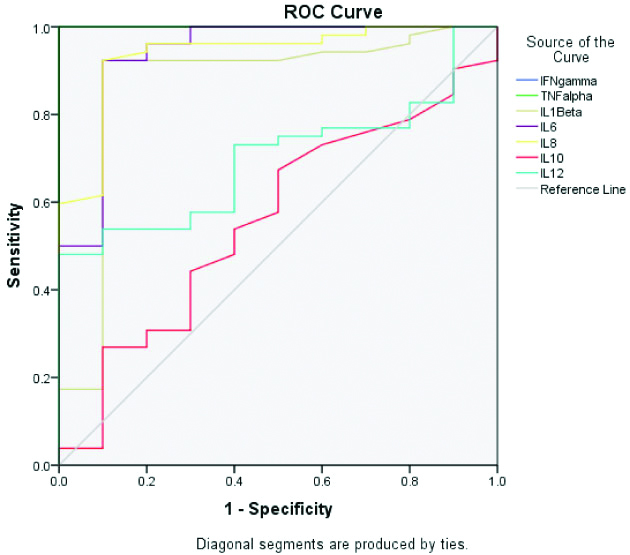
AUC of different serum cytokine concentrations comparing IE cases with controls.
| Area under the curve |
|---|
| IE vs Healthy controls | IE vs Non-IE controls |
|---|
| Test result variable (s) | Area | Std. error | p-value | 95% Confidence interval | Area | Std. error | p-value | 95% Confidence interval |
|---|
| Lower bound | Upper bound | Lower bound | Upper bound |
|---|
| IFN-γ | 1 | 0 | 0.0001 | 1 | 1.00 | 1 | 0 | 0.0001 | 1 | 1 |
| TNF-α | 1 | 0 | 0.0001 | 1 | 1.00 | 0.555 | 0.093 | 0.585 | 0.372 | 0.737 |
| IL-1β | 0.868 | 0.078 | 0.0001 | 0.715 | 1.00 | 0.626 | 0.101 | 0.21 | 0.428 | 0.824 |
| IL6 | 0.938 | 0.049 | 0.0001 | 0.843 | 1.00 | 0.8 | 0.09 | 0.003 | 0.623 | 0.977 |
| IL8 | 0.937 | 0.04 | 0.0001 | 0.857 | 1.00 | 0.876 | 0.076 | 0.0001 | 0.727 | 1 |
| IL10 | 0.559 | 0.096 | 0.559 | 0.371 | 0.747 | 0.435 | 0.094 | 0.515 | 0.251 | 0.618 |
| IL12 | 0.698 | 0.07 | 0.049 | 0.561 | 0.835 | 0.533 | 0.114 | 0.745 | 0.31 | 0.755 |
Greater AUC was seen in IFN-γ, IL-6 and IL-8 levels when IE cases were compared with Non-IE controls [Table/Fig-6,7]. The IL-8 values at an optimal cut-off level of 258.6 pg/mL gave sensitivity of 92.3%, specificity 90%, PPV 97.96% (88.19%-99.68%) and NPV 69.23% (46.18%-85.51%). The IL-6 values at an optimal cut-off level 68.5 pg/mL gave sensitivity 82.7%, specificity 80%, PPV 95.56% (86.08%-98.68%) and NPV 47.06% (31.26%-63.47%).
ROC for different serum cytokine concentrations comparing IE patients with non-IE patients.
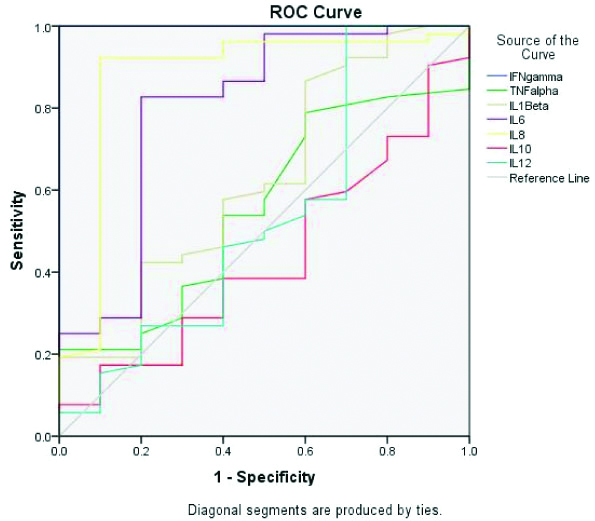
Values of AUC of IFN-γ and TNF-α showed hypothetical value which may be due to sampling variation and hence could not be considered.
Discussion
IE is a serious life threatening disease and the diagnosis is made based on modified Duke criteria which include conventional blood culture and echocardiography as major criteria and a combination of clinical symptoms, vascular and immunological phenomena as minor criteria.
In the present study, which involves 52 IE patients, the major predisposing factor of IE was identified as rheumatic heart disease (40.4%) which is closely in line with the other studies conducted in the Northern (37.7%) and Southern (40.6%) parts of India [3,17].
The commonest causative organisms of culture positive endocarditis in the study were Staphylococcusaureus and Enterococcusfaecalis. Review of previous Indian studies by Garg N et al., and Senthilkumar S et al., reported higher incidence of Streptococcus as aetiological agent [8,18]. However, Ghosh S et al., observed an increased incidence of Staphylococcus species as causative agent of infective endocarditis [19].
The common presenting symptoms of the IE patients in the study were fever (65.4%) followed by breathlessness (53.8%) which is in concurrence with other studies [3,19].
The pathology of IE is influenced by both infection and resulting inflammation. Hence, there is a possibility for variation in levels of inflammatory markers. This along with microbiological diagnosis could aid in reducing the time taken for diagnosis and consequently would aid in better management of IE [12]. Cytokines are a functional class of small protein inflammatory mediators which play key role in initiating and maintaining inflammatory response in sepsis cases [20].
The present study showed higher serum levels of IFN-γ, TNF-α, IL-1β, IL-6 and IL-8 in IE patients when compared with control groups. ROC analysis suggested that IL-6 and IL-8 may be a reliable marker for the diagnosis of IE.
Earlier studies also show similar observations with variations in some cytokine levels. Rawczynska-Englert I et al., had observed a high IL-6 value in IE patients in comparison with two control groups (Rheumatic heart disease patients without IE and patients with urinary tract infection) [21]. Alter P et al., and Watkin RW et al., also reported high IL-6 values in IE patients [10,16].
A significantly high value of IL-1β, IL-12 and TNF-α were observed in IE patients on comparison with non-IE patients and healthy controls by Araújo IR et al., [13]. In the present study, IL-8 values were significantly elevated in IE patients when compared with the two control groups, which is in contrast to Araújo IR et al., who reported no significant difference in IL-8 levels in IE patients when compared with non-IE patients.
Immunohistochemistry studies done by Ekdahl C et al., on heart valve biopsy samples of IE patients showed increased inflammation and greater number of IL-8 containing cells in patients with short pre-operative treatment course and it was concluded that increased levels of IL-8 in infected heart valves could be used as a diagnostic marker [22].
Evaluation of various cytokine levels among culture positive cases did not show significant increase in Staphylococcusaureus positive cases which was different from the previous studies done by Nunes MCP et al., and Araújo IR et al., [12,13].
The mean value of anti-inflammatory cytokine IL-10 was very low in S.aureus IE cases indicating that reduction in IL-10 levels could have an impact on pathogenesis. The role of IL-10 has been recognised in the prevention of spread of acute systemic infections caused by S.aureus by its immunoregulatory action on effector T-cells [23]. Study done by Gjertsson I et al., showed that the absence of IL-10 could lead to impaired clearance of bacteria in S.aureus arthritis cases leading to poor prognosis [24].
The study observed elevated levels of pro-inflammatory cytokines like IL-1β, IL-6, IL-8, TNF-α and IFN-γ in both IE and non-IE patient groups. IL-6 and IL-8 were found to be useful in diagnosing IE patients.
Limitation(s)
This study was carried out in patients attending a tertiary care institution which could, contribute to selection bias and it is possible that some of them could have taken antibiotic therapy before their visit to this hospital which could be a limitation of this study.
Conclusion(s)
Measurement of various serum cytokines (IL-1β, IL-6, IL-8, IL-10, IL-12, TNF-α and IFN-γ) showed that the levels of all of them except IL-10 and IL-12 were significantly higher in patients with IE and non-IE infections as compared to healthy controls. ROC analysis of the cytokine levels proved IL-6 and IL-8 to be reliable indicators for the diagnosis of IE. Hence, the study concludes that IL-6 and IL-8 levels may be used in the diagnosis of IE and can be considered as additional markers for inclusion in Duke’s criteria. Further studies are required to relate the levels of cytokines in other specific infectious conditions.