The conjunctiva and cornea together constitute the first line of defence of the eye. Normal conjunctival flora is either exogenous or endogenous in origin. Commensals of conjunctiva play a vital role in the normal functioning of the ocular system by maintaining surface homeostasis and immuno-regulation. They also inhibit the growth of pathogenic bacteria by competing with them for nutrition [1]. These under normal circumstances do not cause any harm. Nevertheless, these microorganisms are a potential source of infections to the eye when there are changes in the micro-environment of ocular surface or systemic illness. Coagulase-Negative Staphylococcus (CONS), is the most common organism isolated from the conjunctiva and also the most common organism implicated in causing endophthalmitis [2,3]. Hence, knowledge regarding the normal flora of conjunctiva is of paramount importance for an ophthalmologist. Though there are studies conducted on the normal conjunctival flora in various parts of the world [2,3], the results cannot be directly applied for the Indian population. Furthermore, such studies must be done periodically owing to the dynamic nature of microbial flora and resistance patterns. The present study is a part of the study comparing microbiome isolated from non-contact lens users and contact lens users (asymptomatic and symptomatic contact lens users). The other part of the study titled “Comparison of microbiome isolated from the conjunctiva, contact lens and lens storage case of symptomatic and asymptomatic contact lens users” has already been published [4].
Currently, contact lenses are increasingly being used for cosmetic or therapeutic purposes. Lack of compliance and poor hygiene towards lens care are strongly associated with microbial contamination and it has been proved to result in eye infections [4-6]. The most feared complication of contact lens use is Microbial Keratitis (MK). Because of their direct contact with the ocular surface, they exert shear stress, cause local trauma and thinning of the cornea. These factors may alter the ocular microbiome and hence may predispose the eye for corneal infiltrations and hence, infections [7]. There is less data available on the ocular microbial flora of contact lens users and their antimicrobial susceptibility patterns.
To best of our knowledge, there are very few studies comparing the microbial flora between the contact lens and non-contact lens users [8]. Hence, the present study aims to analyse the normal conjunctival flora and compare the same with the microbiome of contact lens users.
Materials and Methods
The present study was a case-control study conducted in the Department of Microbiology and Ophthalmology attached to Bangalore medical college and Research Institute, Bangalore, Karnataka, India. Institution ethical clearance was obtained (No:BMCRI/PS/66/2018-19). The duration of the study was for three months starting from June 2018 to September 2018. Informed written consent was obtained from those who volunteered to participate.
A total of 80 individuals (n=160) in two groups of 40 each were included in the study.
Group 1: 40 (n=80) non-contact lens users in the age group 18-35 years which consisted of undergraduates and post-graduate medical students.
Group 2: 40 (n=80) contact lens users in the age group 18-35 years which consisted of undergraduates and post-graduates studying at medical college. The study subjects were daily silicone hydrogel soft contact lens users who wore contact lens for a minimum of eight hours duration every day and disposed off their contact lens every month. The study participants were contact lens users for more than one year.
All the participants were examined by an ophthalmologist using a slit lamp. Individuals with ocular infections, co-existing ocular diseases, antibiotic use within one month and systemic diseases were excluded from the study. The basic demographic details of the patients (Age, sex, education and occupation) and brief history were collected. In total, there were 40 Non-contact lens users (20 males and 20 females) and 40 contact lens users (20 males and 20 females). The mean age of study population was 26 years and consisted of undergraduate (MBBS) and post-graaduate (MD/MS) students.
Collection of conjunctival samples: Conjunctival samples were collected by swabbing the lower conjunctival sac using sterile cotton swabs and this was transferred immediately into Brain Heart Infusion (BHI) broth. Two samples each (left eye and right eye) were collected from 40 non-contact lens users (n=80) and 40 contact lens users (n=80).
Processing of samples: Culture Media (Mac Conkey agar, blood agar and SDA) sterility was ensured by pre-incubating them before sample inoculation. Prior to sample inoculation, BHI broth was incubated to ensure sterility of the same. After 24 hours, incubation at 37°C in BHI broth, the samples were sub-cultured onto the Blood agar, Mac Conkey agar and SDA. The blood agar and Mac Conkey’s agar were incubated at 37°C for 24-48 hrs, while SDA samples were incubated at 25°C and examined daily for the growth of fungi and discarded at the end of three weeks, if found sterile. Organisms grown were identified using standard microbiological techniques [9]. The antimicrobial susceptibility test for bacterial isolates (Staphylococcus aureus and GNB was done by Kirby–Bauer disc diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines 2017 [10]. The Staphylococcus aureus isolates were tested for susceptibility to antibiotics-penicillin (10 units), cefoxitin (30 μg), erythromycin (15 μg), clindamycin (2 μg), azithromycin (15 μg), tetracycline (30 μg), doxycycline (30 μg) and vancomycin (30 μg). GNB isolates were tested for susceptibility to ampicillin (10 μg), amoxicillin (20 μg)+clavulanic acid (10 μg), azithromycin (15 μg), ciprofloxacin (5 μg), imipenem (10 μg), levofloxacin (μg), cefotaxime (30 μg) and ceftriaxone (30 μg).
Statistical Analysis
The data obtained was in the form of percentages and were analysed using appropriate statistical tests-Chi-square analysis (IBM SPSS statistics software version 25.0) and represented using tables and bar graphs.
Results
Microbial flora obtained from Non-contact lens users/Normal conjunctiva (n=80):
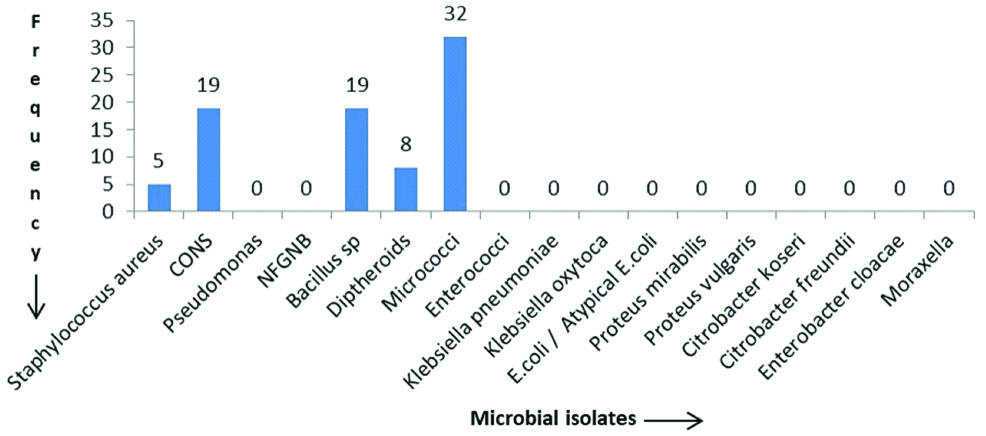
Of the 80 samples collected from non-contact lens users, 78 (97.5%) showed growth on either MacConkey agar or blood agar. None of the samples had growth of fungus. Among the 78 samples which showed growth, five samples exhibited polymicrobial growth (yielding growth of two or more different organisms) and 73 samples had monomicrobial growth. Thus, in total 83 bacterial isolates were obtained. The most common isolate obtained was Micrococcus (38.5%) followed by CONS (22.8%), Bacillus species (22.8 %) and Diptheroids (9.6 %). There were five Staphylococcus aureus isolates of which one was found to be MRSA and four were MSSA isolates which were resistant to penicillin, erythromycin, clindamycin, and azithromycin but sensitive to cefoxitin, tetracycline, doxycycline and vancomycin. There were no GNB obtained. The distribution of microbial isolates is depicted in [Table/Fig-1].
Frequency of microbial isolates from the conjunctiva of non-contact lens users.
Microbial Isolates from the Conjunctiva of Contact Lens Users (n=80)
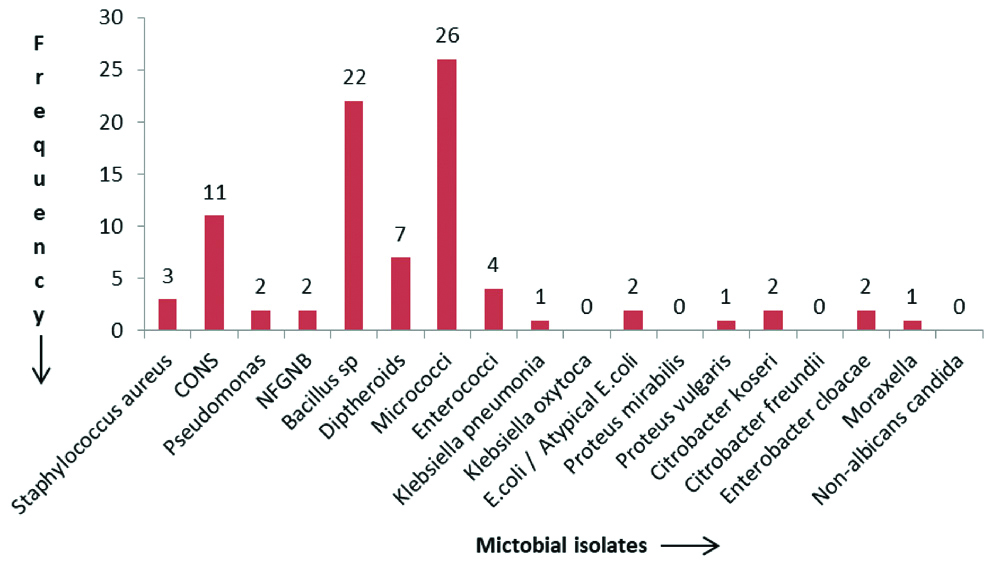
Of the 80 samples collected, 79 (98.7%) showed growth on either MacConkey agar or blood agar. None of the samples had growth of fungus. Among the 79 samples which showed growth, six samples exhibited polymicrobial growth and 73 samples had monomicrobial growth. Thus, in total, 86 bacterial isolates were obtained. The commonest isolate obtained was Micrococcus (30.2%) followed by, Bacillus species (25.5%), CONS (12.7%) and Diphtheroids (8.1%). There were three Staphylococcus aureus isolates of which one was found to be MRSA. The MSSA isolates were resistant to penicillin, erythromycin, clindamycin, and azithromycin but sensitive to cefoxitin, tetracycline, doxycycline and vancomycin. The distribution of microbial isolates is depicted in [Table/Fig-2].
Frequency of microbial isolates from the conjunctiva of contact lens users.
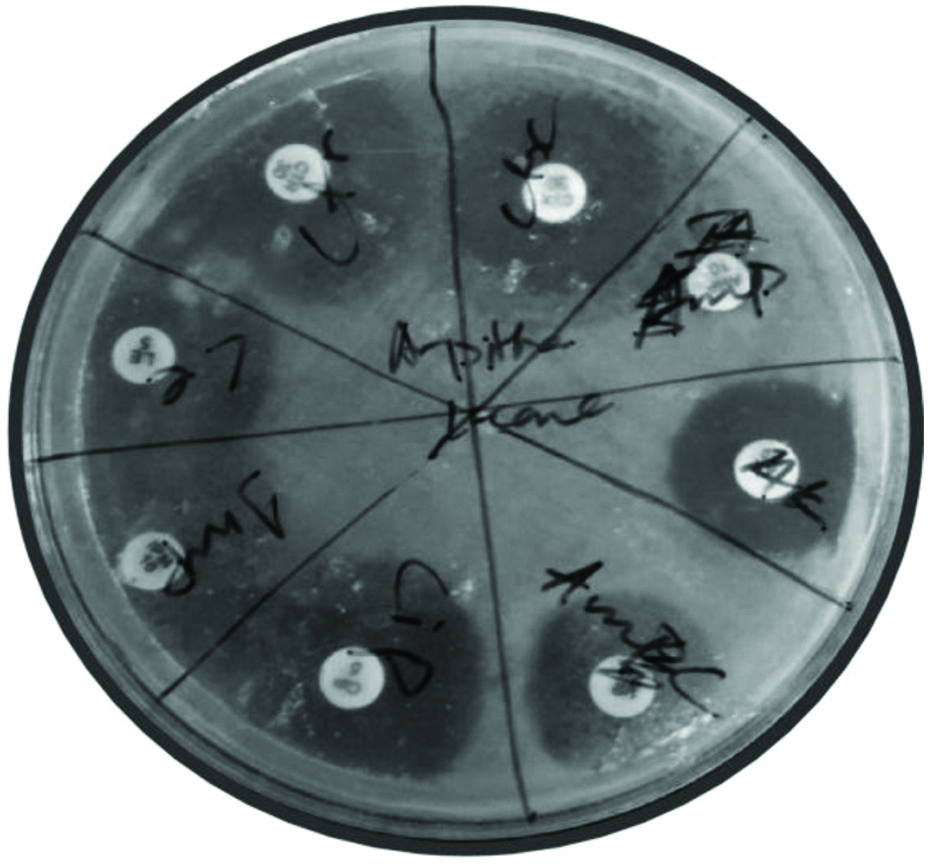
There were 12 GNB found [Table/Fig-3]. Of them 10 were ampicillin resistant, seven were amoxicillin-clavulanate resistant and three were cefotaxime resistant (p-value=0.012) [Table/Fig-4], but they were all sensitive to ceftriaxone, imipenem, levofloxacin, amikacin and ciprofloxacin. The petri dish plates showing antimicrobial susceptibility test is depicted in [Table/Fig-5].
Distribution of gram negative bacilli from the conjunctiva of contact lens users.
| Organism | Frequency | Percentage |
|---|
| Pseudomonas | 2 | 2.3 |
| NFGNB | 2 | 2.3 |
| Klebsiella pneumoniae | 1 | 1.1 |
| E.coli/Atypical E.coli | 2 | 2.3 |
| Proteus vulgaris | 1 | 1.1 |
| Citrobacter koseri | 2 | 2.3 |
| Enterobacter cloacae | 2 | 2.3 |
| Total | 12 | |
Antimicrobial susceptibility pattern of gram negative bacilli (Chi-square analysis).
| Antibiotic | Resistant | Sensitive |
|---|
| Ampicillin | 10 | 2 |
| Amoxicillin+Clavulanate | 7 | 5 |
| Cefotaxime | 3 | 9 |
p=0.012*, Significant, Chi-Square Test
Petridish showing antimicrobial susceptibility test for Klebsiella pneumoniae isolated from lens case. Antibiotic discs used are- ampicillin (10 μg), amoxicillin (20 μg)+clavulanic acid (10 μg), azithromycin (15 μg), ciprofloxacin (5 μg), imipenem (10 μg), levofloxacin (μg), cefotaxime (30 μg) and ceftriaxone (30 μg).
The antibiogram of MSSA isolates in both the groups (contact lens and non-contact lens) is shown in [Table/Fig-6].
Antimicrobial susceptibility pattern result of MSSA isolates.
| Antibiotic | Sensitive/Resistant |
|---|
| Penicillin | Resistant |
| Clindamycin | Resistant |
| Azithromycin | Resistant |
| Erythromycin | Resistant |
| Cefoxitin | Sensitive |
| Tetracycline | Sensitive |
| Vancomycin | Sensitive |
| Doxycycline | Sensitive |
Comparison of Conjunctival Flora between the Contact Lens and Non-contact Lens Users
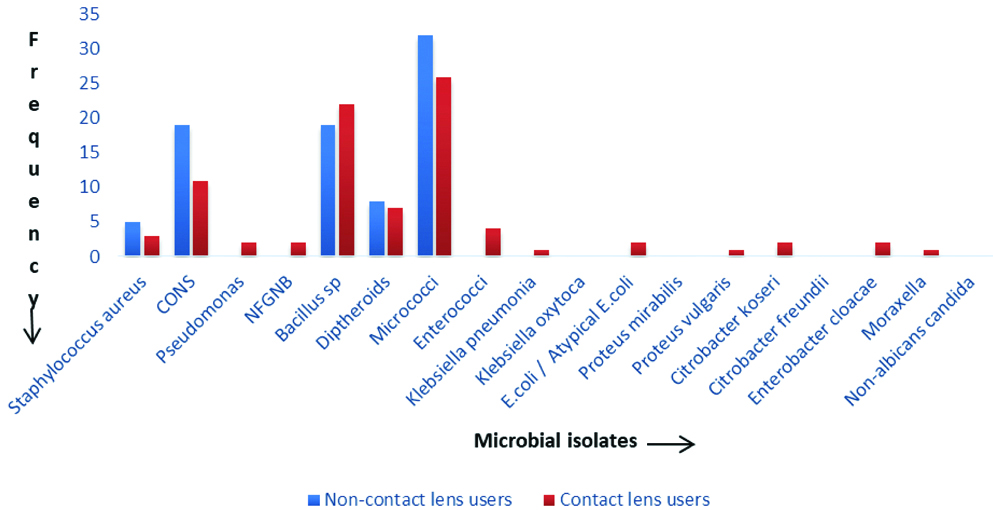
Polymicrobial growth was seen in five samples in non-contact lens users and in six samples in contact lens users. GNB including Non-fermenter GNB (NFGNB) (2.3%), Pseudomonas (2.3%), E.coli (2.3%), Enterobacter cloacae (2.3%), Citrobacter koseri (2.3%), Proteus vulgaris (1.1%) and Klebsiella pneumoniae (1.1.%) were isolated only from the conjunctiva of contact lens users and none from non-contact lens users (p-value <0.001) [Table/Fig-7].
Chi-square analysis of GNB between non-contact lens users and contact lens users.
| Gram Negative Bacteria (GNB) | Non-contact lens users | Contact lens users |
|---|
| No of isolates present | 0 (0%) | 12 (13.9%) |
| No of isolates absent | 83 (100%) | 74 (86.04%) |
p<0.001**, Significant, Chi-Square Test
However, the percentage of CONS obtained was higher in non-contact lens users. There is little or no difference in the percentage of Micrococcus, Bacillus species, Staphylococcus aureus, MRSA obtained from non-contact lens users and contact lens users. The comparison of isolates obtained from non-contact lens and contact lens users is depicted in [Table/Fig-8].
Comparison of conjunctival flora between the contact lens and non-contact lens users.
Discussion
The knowledge concerning normal conjunctival flora is important for understanding the pathogenesis of ocular infections and appropriate administration of prophylactic antibiotics. The high percentage of growth found in both non-contact lens (97.5%) and contact lens users (98.7%) may be attributed to the fact that in the present study, samples were immediately transferred into BHI broth without any delay and were subcultured onto blood agar, MacConkey and SDA after 24 hours of incubation.
In the present study, the frequency of sterile conjunctival sac obtained in non-contact lens users and contact lens users was 2.5% and 1.3%, respectively. The percentages of the sterile conjunctival sac in other studies on the analysis of normal conjunctival microbial flora show great difference amongst them. Locatcher-Khorazo D and Seegal BC [11] reported 0% sterile conjunctival sac whereas other studies by Debnath SC, Smith CH and Starr MB and Lally JM reported the frequency of sterile conjunctival sac to be 30% and 47% respectively [12-14].
A study conducted by Fernández-Rubio E et al., on non-contact lens users/normal conjunctival flora, reported a higher prevalence of CONS in 88.3%, followed by Diphtheroids in 58.1%, Propionibacteria in 31%, Streptococcus in 23.1%, Staphylococcus aureus 10.2%, Haemophilus plus Gram-negative diplococci 7.5% and other Gram-negative rods 4.5%, Enterococcus 2% [15].
Micrococcus was the most common isolate found in both groups (non-contact lens and contact lens users) followed by CONS, Bacillus species and Diphtheroids in this study which differs from other studies on the normal microbial flora of the conjunctiva [2] and contact lens users [16] where the most common isolate was CONS. There were no fungal isolates obtained in non-contact lens users as well as contact lens users in comparison to studies conducted by Smith CH on normal conjunctival flora and Mela EK et al., on contact lens users where fungal isolates have been isolated [13,17]. The presence of MRSA isolates among both groups may be due to the study population being exposed to the hospital environment. The MSSA isolates in both the groups were resistant to penicillin, erythromycin, clindamycin, and azithromycin but sensitive to cefoxitin, tetracycline, doxycycline and vancomycin. Gram negative bacteria including NFGNB, Pseudomonas, E.coli, Enterobacter cloacae, Citrobacter koseri, Proteus vulgaris, Klebsiella pneumoniae and Moraxella were exclusively found in the conjunctiva of contact lens users (p-value=<0.001). The present study included individuals who wore contact lens for the duration of minimum one year. Hence findings of the long-term effect of contact lens wear could derive. More than half of contact lens users reported symptoms of dryness, lacrimation, redness, itching or foreign body sensation which is similar to study conducted by Unnikrishnan B and Hussain S, [18]. The association of altered ocular microbiome in contact lens users and the aforementioned symptoms requires follow-up studies and analysis of the microbial flora from the contact lens and its accessories, amikacin and ciprofloxacin. Hence, the present study helped in formulating antibiotic policy (prophylactic use and empiric treatment) for our healthcare establishment in both contact lens users and non-contact lens users. Similar studies, if done at regular intervals, can help in customising the antibiotic policy in respective healthcare settings.
Limitation(s)
Follow-up investigations on subjects whose conjunctival swabs grew GNB was not done. This could have helped to determine whether the obtained isolates are a part of transient flora or resident flora of the conjunctiva.
Conclusion(s)
The present study shows that the use of contact lens significantly alters the normal conjunctival flora and are a potential source of infection. There were significant number of bacteria that were ampicillin, amoxicillin-clavulanate and cefotaxime resistant. Further studies consisting of larger sample size, study population of people working in both healthcare and non-healthcare associated occupations, various types of contact lenses, follow-up studies are recommended to obtain a more objective and clear picture of conjunctival flora and its changes on usage of contact lens.