Lead (Pb) is a ubiquitous, non-biodegradable, environmental chemical and occupational contaminant that is widely distributed around the world [1]. It possesses some unique physical and chemical properties viz. malleability, ductility, anti-corrosiveness, poor conductivity and softness, by the virtue of which Pb finds its application in various industries worldwide, since time immemorial [2]. Currently Pb is used in industries such as battery manufacturing, smelting, jewellery making, mining, paints, ceramics, porcelain dyes, rubber and folk remedies [3]. Due to the widespread use of Pb in such industries, occupational exposure to Pb among the workers can cause significant toxic effects in their central nervous, haematopoietic, renal, gastrointestinal, cardiovascular and reproductive systems. [4]. Moreover there is a growing degree of evidence that, long term Pb exposure may contribute to an increased risk of cancer development [5]. The International Agency for Research on Cancer has classified inorganic Pb compounds into Group 2A of probable human carcinogens [6]. A few investigations on Pb exposed subjects showed an association between enhanced risk of cancers of the stomach, lung and bladder, and exposure to Pb [7-9]. Occupational exposure to Pb has been found to be associated with approximately 2-8% of all such cancer cases [10].
Mechanisms by which Pb can cause cancer are still unclear, however various possible mechanisms have been proposed regarding carcinogenic properties of Pb, which may act at cellular or molecular level. Pb can induce DNA damage through direct or indirect interactions and thereby enhance or promote the process of carcinogenesis. Pb can inhibit the activity of many enzymes and contributes to oxidative stress, increases rate of DNA single and double strand breaks, DNA protein crosslinks, induces micronuclei formation, chromosomal aberrations and causes DNA damage [11-13]. Moreover, Pb can enhance the genotoxicity of other DNA damaging agents (such as UV light, X-rays and certain chemicals) and thus act as co-mutagen, predominantly by interfering with DNA replication fidelity and repair processes [11,14]. Pb can also alter chromosome segregation because it interacts with cytoskeleton proteins [1].
Pb plays an important role in various small and large scale industrial enterprises in India; hence bio-monitoring of occupationally exposed subjects becomes imperative. In order to reduce the exposure and carcinogenic risk, identification of the adverse effects at the earliest is crucial; hence the goal of the present study was to evaluate the blood Pb levels and the associated DNA damage among the workers in Jaipur, Rajasthan, India who were occupationally exposed to Pb.
Materials and Methods
This cross-sectional, case-control study was conducted in Department of Biochemistry, Jaipur National University Institute of Medical Sciences and Research Centre, Jaipur a tertiary care center in Jaipur, Rajasthan, India for a period of three years from January 2016 to December 2018. Prior to the collection of blood samples, approval from Institutional Ethical Committee (IEC) was obtained (ECR/905/Inst/RJ/2015).
Study Population
The study protocol and the objectives of the study were explained to the enrolled subjects and their written informed consent was obtained. The selection of the subjects was based on predesigned questionnaire [Appendix 1] including demographic details, medical history (regarding medications, vaccinations, exposure to X-rays), lifestyle factors (smoking, chewing tobacco, alcohol intake) and occupational exposure to Pb (working hours/day, years of exposure, use of protective gear etc.,). A total of 220 subjects were included in the study (110 in each, Pb-exposed group and control group).
Pb-exposed group
The subjects included in this group were building construction workers including the ones involved in tiles and granite cutting, painters, motor garage workers, denting and painting workers, battery workers involved in removing Pb electrodes, smelting, recycling of Pb batteries and manufacturing and assembling Pb-acid storage batteries. The age of Pb-exposed subjects ranged from 17 to 46 years and they were exposed to Pb from 2 to 15 years, with a daily exposure ranging from 4 to 10 hours.
Control Group
The subjects included in this group were also selected based on the questionnaire and comprised of the general population without any history of exposure to Pb or any known physical or chemical agent in their workplace, however, they belonged to the same age group and socioeconomic status as that of the Pb-exposed group.
Confounding factors like age, alcohol consumption; smoking and duration of working years and their effects on blood Pb levels and DNA damage were also investigated. The subjects who smoked >5 cigarettes or bidi/day or chewed tobacco atleast 5 times/day and who took 5 glasses of an alcoholic drink/day for atleast past 12 months were considered as smokers and alcoholics respectively, in both Pb-exposed and control groups.
Sample Collection
Blood samples were collected from the Pb-exposed subjects and control subjects and were coded to avoid possible bias. A total of 15 mL venous blood was collected from each subject by using sterilised syringes. From 15 mL, a total of 5 mL blood was dispensed into a heparinised vacuum tube and was used for comet assay. Another 5 mL blood was dispensed into EDTA vacuum tube for estimation of blood Pb level. The remaining 5 mL was dispensed in a plain vial without anticoagulant to extract serum after centrifugation at 2,500 rpm for 10 minutes. The serum was then collected in eppendorf tubes and was stored at -20°C for further investigations. The samples were transported to laboratory in a leak proof box with cool packs and were processed within two hours after collection.
Blood Lead Estimation
The blood Pb levels were quantified by using an inductively coupled plasma mass spectrometry with triple quadropole technology (iCAPTM TQ ICP-MS, Thermo Fisher Scientific, Bremen, Germany). The EDTA blood was homogenised for 10 minutes by mechanical shaking; thereafter blood plasma (1.0 mL) was gravimetrically diluted (in pre-cleaned polypropylene bottles kept in 2% nitric acid for 72 hours) with 0.5% m/m nitric acid (Fisher Scientific) and 2% m/m tetramethylammonium hydroxide (Merck, Sigma Aldrich) in ultrapure water. A calibration blank, a series of standards and a quality control were prepared by using the same procedure. All samples and standards were spiked with an internal standard mix (10 μg/L of 209Bi). The sample digests were filtered with Whatman paper several times to obtain a clear solution. The diluted digests were measured directly by ICP-MS, and concentration of Pb in blood was quantified as microgram per deciliter.
Comet Assay
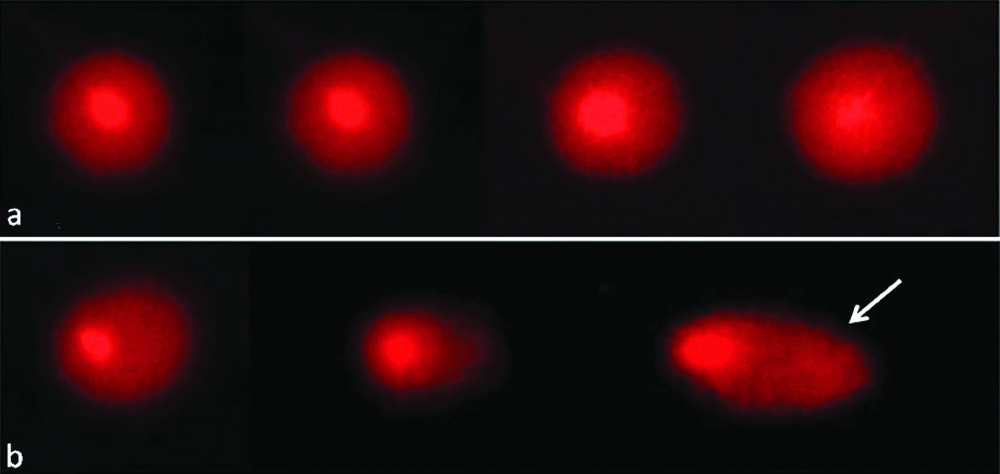
The DNA damage was analysed in blood lymphocytes using alkaline comet assay according to the method by Singh NP et al., with minor modifications and under low brightness, due to the photosensitivity of the assay [15]. Cell viability was determined by the trypnan blue exclusion technique and ranged from 92-96%. The slides were prepared in duplicate per subject, as per the previously described protocol [16]. Analysis was performed using a 400x magnification using a fluorescence microscope (Olympus BX 51, Japan) equipped with a 515-560 nm excitation filter and a 590 nm barrier filter. Slides were than analysed by using a comet image analysis system, Comet v. 5.5 (Kinetic Imaging Ltd., Nottingham, UK). The coded slides were scored by one person throughout the study, to avoid inter scorer variability. A total of 100 individual lymphocyte cells were screened per subject (50 cells from each slide). An undamaged lymphocyte resembled an intact nucleus without a tail, and a damaged lymphocyte had the appearance of a comet. Comets were selected without bias and represented the whole gel. Comets seen in edges, air bubbles and overlaps were rejected. The DNA in the tail (tail DNA %) was used to evaluate DNA damage.
Statistical Analysis
The coding of the samples was done at the time of the preparation and scoring, and the same were decoded before statistical analysis for comparison. The data were analysed using Statistical Package for Social Sciences (SPSS 17.0). The significance of the differences between controls and exposed subjects endpoint means were analysed using student’s t-test. Mean values and Standard Deviation (SD) were computed for the scores. Student’s t-test was used to determine the statistical significance (p<0.05) of the effects (age, years of exposure, smoking and alcohol consumption). To assess the association between Pb and years of exposure (an independent variable) simple linear regression analysis was performed.
Results
The demographic details of the study subjects of both the groups were summarised by age, years of exposure, daily exposure, smoking habit and alcohol consumption are depicted in [Table/Fig-1]. The mean age of Pb-exposed subjects was 31.72±5.12 years and of control group subjects was 31.91±4.89 years and were almost similar.
Demographic details, blood lead levels and comet assay results of the study participants (n=220).
| Demographic factors | Control group (n=110) | Pb-exposed group (n=110) |
|---|
| Age (years) | 31.91±4.89 | 31.72±5.12 |
| Exposure to Pb (years) | - | 9.57±3.81 |
| Daily exposure to Pb (hours) | - | 5.82±1.69 |
| Smoking/Tobacco chewing |
| Yes | 42 (38.2%) | 91 (83%) |
| No | 68 (61.8%) | 19 (17%) |
| Alcohol consumption |
| Yes | 47 (42.7%) | 89 (80.9%) |
| No | 63 (57.35) | 21 (19.09%) |
| Sex ratio | Male 96 (87%) | Male 96 (87%) |
| Female 14 (13%) | Female 14 (13%) |
| Blood investigations |
| Blood lead levels (μg/dL) | 4.89±1.93 | 38.03±12.92* |
| Comet assay (% DNA-T in μm) | 6.12±1.80 | 14.80±1.31* |
BLL: Blood lead level; %DNA-T: percentage of DNA in the tail.
Data represented as mean±standard deviation. Significantly different from control at *p<0.01 (Student’s t-test)
The mean blood Pb levels (in μg/dL) of exposed group (38.03±12.92) were found to be significantly higher than those of control group (4.89±1.93) and are depicted in [Table/Fig-1]. The level of DNA damage in lymphocytes of study subjects was analysed by comet assay. The mean % of tail DNA significantly increased in lymphocytes of exposed group when compared to controls (14.80±1.31 vs. 6.12±1.80) [Table/Fig-1,2].
Level of DNA damage using comet assay. (a) Normal lymphocytes with intact DNA; (b) Exposed lymphocytes with varying degree of DNA fragmentation, showing migrating tail DNA.
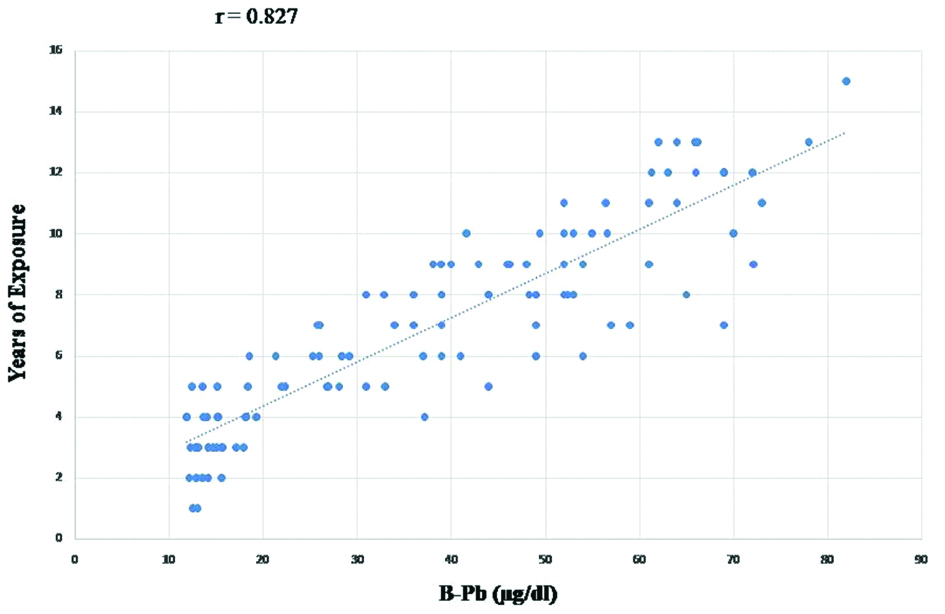
Analysis of blood Pb values and the level of DNA damage showed that they were significantly different for smokers in comparison to non-smokers in the exposed group [Table/Fig-3]. The blood Pb levels of the exposed subjects positively correlated with years of exposure (r=0.827, R2=0.685, p<0.05; highly significant) when compared to control groups [Table/Fig-4]. Moreover, higher mean DNA damage was found in subjects with prolonged exposure to Pb [Table/Fig-3]. Age and alcohol consumption did not show any effect on blood-Pb levels and the level of DNA damage in both the groups.
Blood lead level and DNA damage with respect to smoking, alcohol consumption and years of exposure in control group and Pb-exposed group.
| Characteristics | n (%) | Blood-Pb level (μg/dL) | Comet Assay (% DNA-T (in μm) |
|---|
| Control Group (n=110) |
| Smoking |
| Yes | 42 (38.2%) | 5.42±0.83 | 7.53±0.83 |
| No | 68 (61.8%) | 5.07±0.91 | 6.94±0.97 |
| Alcohol consumption |
| Yes | 47 (42.7%) | 5.18±0.86 | 6.51±0.91 |
| No | 63 (57.35) | 5.23±090 | 5.93±0.84 |
| Pb-Exposed Group (n=110) |
| Smoking |
| Yes | 91 (83%) | 44.19±4.56* | 15.71±1.06* |
| No | 19 (17%) | 37.37±5.28 | 13.55±1.13 |
| Alcohol consumption |
| Yes | 89 (80.9%) | 41.72±3.51 | 15.63±0.73 |
| No | 21 (19.09%) | 39.61±4.13 | 13.92±0.86 |
| Years of Exposure |
| ≥8 years | 72 (65.45%) | 47.81±2.89* | 15.97±1.37* |
| ≤8 years | 38(34.55%) | 39.37±4.62 | 13.35±1.14 |
Effect of confounding variables (Smoking, Alcohol and Years of exposure) within control and exposed groups. Data represented as mean±standard deviation. Significantly different at *p<0.05 (Student’s t-test)
Fitted line curve between Blood-Pb levels and years of exposure.
Discussion
Despite technological developments, exposure to Pb remains a major public health concern particularly among the workers occupationally exposed to Pb. After inhalation, oral or dermal exposure, Pb is readily absorbed in the body. Although the absorbtion through dermal exposure is less efficient but the other two routes are the predomominant sources of exposure among the occupationally exposed workers [17]. Out of the total inhaled Pb, approximately 30-40% is absorbed into the bloodstream whereas gastrointestinal absorption depends on the nutritional status and age [1]. After absorption into the bloodstream, most of the Pb is carried, bound to erythrocytes. The freely diffusible plasma fraction of the Pb is extensively distributed throughout tissues, and is present in high concentrations in bone, teeth, liver, lungs, kidneys, brain and spleen [18]. Lead is excreted mainly by renal and gastrointestinal pathways, but the excretion is quite slow and hence accumulation in the body occurs easily, which eventually leads to multisystem toxicity even at very low levels of exposure.
Present study results revealed that blood-Pb levels among the Pb exposed subjects were significantly increased in comparison to the control subjects. Blood Pb levels have a median biological half life of approximately 30-35 days and is an indicator of the current exposure. It generally reflects the dynamic equilibrium between absorption and disruption of Pb in the body and its elimination from the body. Bone Pb levels, have a half life of around 25-30 years and signify the accumulated exposure [18]. However, Pb concentration in blood increases with years of exposure and hence the determination of blood-Pb levels is one of the most sensitive biomarkers for Pb exposure [19]. The biological exposure index for blood-Pb levels is listed as 30 μg/dL [4], however the mean blood-Pb levels of the Pb exposed subjects in present study were found to be significantly higher than the prescribed limits. Present study findings were parallel to the previous studies, where the high blood-Pb levels were reported among the automobile battery recycling workers in comparison to the controls [17,20,21].
A significant increase in blood-Pb level observed in the present study may have resulted in genetic material damage, as assessed by alkaline comet assay. Steinmetz-Beck A et al., suggested a positive linear correlation between the blood-Pb concentrations and the values of comet assay [22]. Comet assay is widely used to assess the genotoxic effects of Pb in occupationally exposed subjects, and can effectively and efficiently detect the DNA single strand breaks and alkali labile sites [1,11,12,17,21-23]. In the present study, the comet assay demonstrated significant increase in the level of DNA damage among the Pb-exposed subjects when compared with controls. Occupational exposure to Pb is associated with decreased repair capacity of DNA [10]. Higher blood Pb concentrations can cause increase in comet parameters, which is probably due to prolonged exposure to Pb, less efficient and more error prone mechanisms of DNA repair. Present study findings were parallel with the researchers from Croatia, Brazil, Portugal and India [11,21,24,25], who also observed markedly higher DNA damage among the Pb-exposed workers in comparison to control subjects. However, in contrast to present findings, a study from Portugal did not show any significant difference in the comet assay parameters between the Pb-exposed and control groups [26]. Various animal studies used comet assay to evaluate the DNA damage caused due to Pb poisoning. Different animal species (at varying stages of development) were poisoned with Pb salts (orally and parentally). Although different protocols were followed for these experimental studies, the results regarding the ability of Pb to induce DNA damage were consistent. The level of damage related to the Pb dosage, time of exposure and the examined tissues [16,27-29].
Various in vivo and in vitro studies have used methods other than comet assay to investigate the Pb induced genotoxicity. The results of these studies were discordant, whereas the results with comet assay were much more consistent [2]. Different types of DNA damage can be detected using various cytogenetic tests that mainly detect the cumulative DNA damage whereas comet assay detects the type of DNA damage which can be easily repaired and reflects recent exposures [10]. Selection of different protocols to detect DNA damage might be the possible reason for the discordant results [10].
We also investigated various confounding factors viz. age, alcohol consumption, smoking and duration of working years. We observed that smoking and duration of exposure (in years) caused significant increase in blood Pb levels as well as the level of DNA damage. Present study findings were in tandem with various other researchers who also found positive correlation of smoking and years of Pb-exposure with the blood-Pb levels and level of DNA damage [11,17,20,30]. A meta-analysis of 38 studies also indicated the strong association between smoking habits and level of DNA damage [31]. We did not find any significant correlation of age and alcohol consumption with the blood-Pb levels and the DNA damage.
Present study results clearly indicate a direct relationship between Pb exposure and induction of genetic damage. There are very few studies on lead induced genotoxicity from India [16,20,25], particularly from Rajasthan. Occupationally, exposed workers in automobile repair workshops, denting-painting workshops, battery workers, painters, carpenters and building construction workers comprised the Pb-exposed group of present study. Majority of these workers have a poor socioeconomic status and are considered as high-risk group to the adverse effects of Pb exposure. However most of these workers are oblivious of the hazards they are exposed to, probably due to the illiteracy or lack of awareness programs and hence pay little or no attention in protecting them from possible ingestion or inhalation of the toxic metal [16,20,25,32]. Moreover, para-occupational Pb exposure to children can also occur as their parents are working in such enterprises [33]. Pb exposure in children is of particular concern, due to the rapid absorption, moreover the developing nervous system of children is highly susceptible to the adverse effects of the Pb. Elevated blood Pb levels in children can result in learning disabilities, behavioural problems and mental retardation [33].
Regrettably, in many developing countries, including India, occupational exposure to Pb is not properly regulated or monitored. Many of such establishments are unregistered or unlicensed and are rarely inspected, which accounts for increased occupational exposure to Pb [21,25,32]. Moreover, due to the economic constrains, lack of employment opportunities and social conditions, and the desire to work with the same job only, most of these workers continue to work without the suitable protective gears like respiratory dust masks, clean clothing, etc., and are exposed to this toxic metal for quite long.
Conclusion(s)
The data extrapolated from present study emphasises the necessity of surveillance in exposed workers. A multifaceted approach is needed for effective and efficient preventive actions to be taken, improving the working conditions and ensuring better safety measures to minimise the occupational exposure of the workers. As the associated deleterious effects of Pb-exposure can be serious, so we propose that a routine-periodical screening of the workers exposed to Pb should be conducted. Those who are found to have blood Pb levels over and above the prescribed upper limit should be briefed about the health hazards associated with Pb-exposure and smoking/tobacco chewing as one of the important contributory factor for the same. Health education and awareness programs related to Pb exposure and the associated health hazards should be organised for the occupationally exposed workers. Present study results can serve as a template for the policy makers who should look into this regard and tailor the guidelines accordingly for the timely implementation of the required preventive measures. Remember that it’s better to ‘prevent and prepare, rather than repent and repair.’
BLL: Blood lead level; %DNA-T: percentage of DNA in the tail.
Data represented as mean±standard deviation. Significantly different from control at *p<0.01 (Student’s t-test)
Effect of confounding variables (Smoking, Alcohol and Years of exposure) within control and exposed groups. Data represented as mean±standard deviation. Significantly different at *p<0.05 (Student’s t-test)