To reach an aesthetically pleasing and harmonious smile, current dentistry offers tooth-bleaching techniques that cause a positive psychosocial impact on patients [1]. Several bleaching methods were introduced over the last years. The most common one is the at-home treatment using 10%, 16% or 22% carbamide peroxide [2]. In this technique, the bleaching agent is applied with individual trays, which minimises the direct contact between the gel and the oral mucosa; however, a certain amount of bleaching gel may contact the gingiva causing potential damage [3]. There is evidence that carbamide peroxide at concentrations of 10% and 16% is not genotoxic to the oral mucosa [4,5], however the effects of 22% gel on the cells is unknown.
Genotoxicity tests have simple execution and could be extensively applied for different dental products [6-8]. The MN methodology is frequently applied in genotoxicity tests, which consists of assessing the frequency of whole chromosomes (aneugenic damages) or chromosome fragments (clastogenic damages), which fail to bond to the main nucleus, producing a smaller secondary one- the micronucleus itself [9].
Thus, this study evaluated the genotoxic effects of 22% carbamide peroxide on the human mucosal cells using the MN technique.
Materials and Methods
The present study is an observational clinical-laboratory experiment conducted between March and November 2019 at the Dentistry School and the Biological Sciences Institute of the University of Passo Fundo (UPF), RS, Brazil. The Research Ethics Committee of the UPF approved this study, under protocol #889.508/2014.
This study used a convenience sample of 16 volunteers (7 men and 9 women, aging from 20 to 26-year-old). Inclusion criteria were: healthy participants aging from 20 to 30-year-old who had never performed tooth bleaching. Exclusion criteria were: patients undergoing orthodontic treatment; volunteers presenting with dentinal hypersensitivity, signs of mucosal lesions, caries or gingival inflammation [4,10], carious/non-carious cervical lesions, fluorosis or enamel defects, and endodontically treated anterior teeth. The volunteers having systemic health problems (previous exposure to chemicals, excessive solar radiation or radiotherapy; pregnancy/lactation or use of any medication that could cause tooth discolouration) and/or habits that might interfere with the study, such as smoking and/or alcoholism were also excluded [4,5].
All the 16 patients included in the study lived in the same geographic region (Planalto Médio Sul Riograndense, RS, Brazil) and had similar dietary habits. Baseline values calculated from the cells collected before bleaching were taken as the control values for each volunteer. Thus, at the end of the study, the volunteers themselves served as their own controls.
Tooth bleaching: Alginate (Jeltrate™, Dentsply, York Country, Pennsylvania, United States) impressions of the volunteers’ maxillary and mandibular dental arches were made to produce the acetate trays with one: (1) mm thickness without deposits [Table/Fig-1a]. Each volunteer received the trays and three syringes of 22% carbamide peroxide (Whiteness Perfect™, FGM, Joinville, SC, Brazil). Instructions for the use of bleaching agent were provided: the whitening gel should be dispensed within the whitening trays at the buccal surface of the upper and lower teeth, from the incisal edge to the cervical margin [Table/Fig-1b]. Instructions were also given to the patients regarding oral hygiene (brushing 3 times a day using a non-staining toothpaste) [11]. The treatment was performed daily, two hours a day, for 21 days [Table/Fig-1c].
a) Upper and lower acetate trays made in a vacuum laminator based on the patients’ dental arch models; b) Adequate amount of gel dispensed within the whitening trays at the buccal surface of the teeth, from the incisal edge to the cervical margin; c) Study volunteer with trays positioned in the dental arches; d) Collection of marginal gingiva mucosal cells using a wooden spatula in a participant of the study.
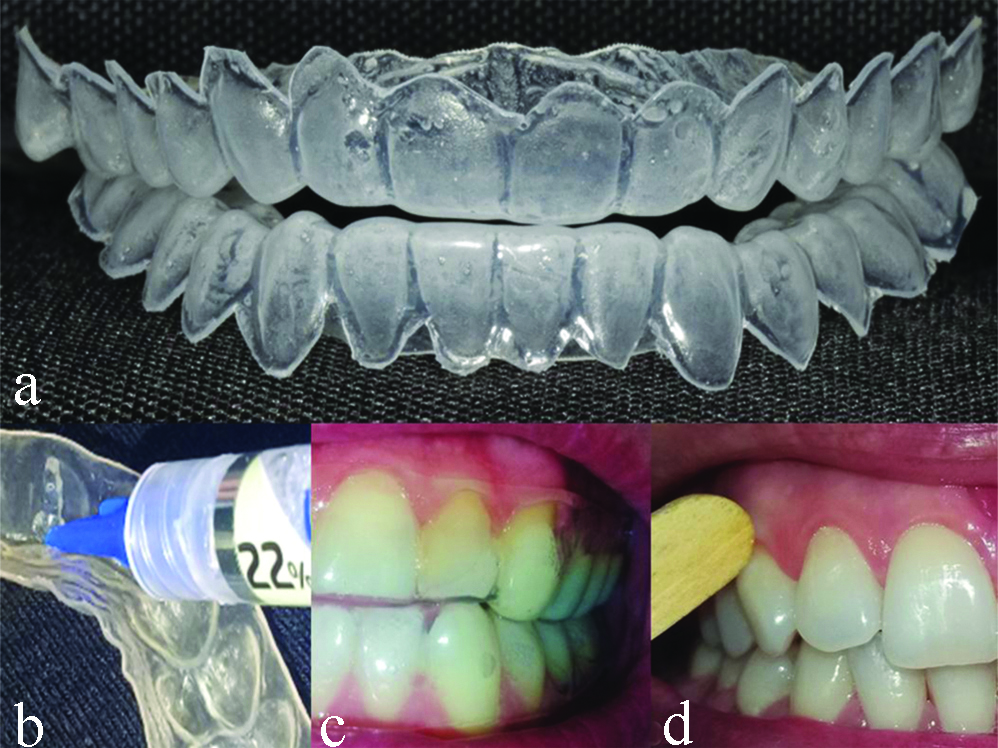
Cytology: The oral mucosa next to the sites of bleaching agent application (gingival margin) was scraped at four different times for cell collection. The first collection was performed at day 0 (D0=baseline/control), before the bleaching treatment. The second collection occurred at day 14 (D14), two weeks after starting the bleaching treatment. The third collection was performed at day 21 (D21- last day of tooth bleaching). The fourth (last) collection occurred at day 52 (D52), 31 days after finishing tooth bleaching.
The methodology was based on previous studies [4,12,13] which demonstrated that the presence of micronuclei/metanuclear alterations in exfoliated cells of the oral mucosa reflect the genotoxic events that occurred in cells of the dividing basal layer of the epithelium 1-3 weeks before the scrapings (cell turnover).
After rinsing the volunteer’s mouth with water, cells were collected by scraping the marginal gingival mucosa (the area closest to the bleaching gel application) using a wooden spatula. The scrapings were performed on the marginal gingiva of the upper and lower arches on both sides (right and left) to standardise cell collections [Table/Fig-1d].
The material collected was transferred to a Falcon tube containing fixing solution (methanol:acetic acid at 3:1) and centrifuged at 1000 rpm for 10 minutes. The material was dropped and smeared onto slides with a platinum loop and left for drying overnight. The slide was then subjected to a 15-minute bath in methanol PA (100%) for fixation. The air-dried slides were stained for 40 minutes in 5% Giemsa. Later, the slide was washed with distilled water and air-dried at room temperature. Once dry, the material was analysed under optical microscopy [4,5].
Microscopic and statistical analysis: The slides were examined continuously from right to left in a random order by a blind trained and experienced examiner. A light microscope (Olympus Bx50, Tokyo, Honshu, Japan) was used at 1000× magnification to determine the frequency of cell alterations in a total of 1000 cells from each patient for each collection time, according to the criteria proposed by previous studies [Table/Fig-2] [14-17].
Oral mucosal cells alterations: a) pycnosis; b) karyorrhexis; c) micronuclei; d) binucleated cell; e) broken egg; f) karyolysis (Giemsa Wright, 1000X).
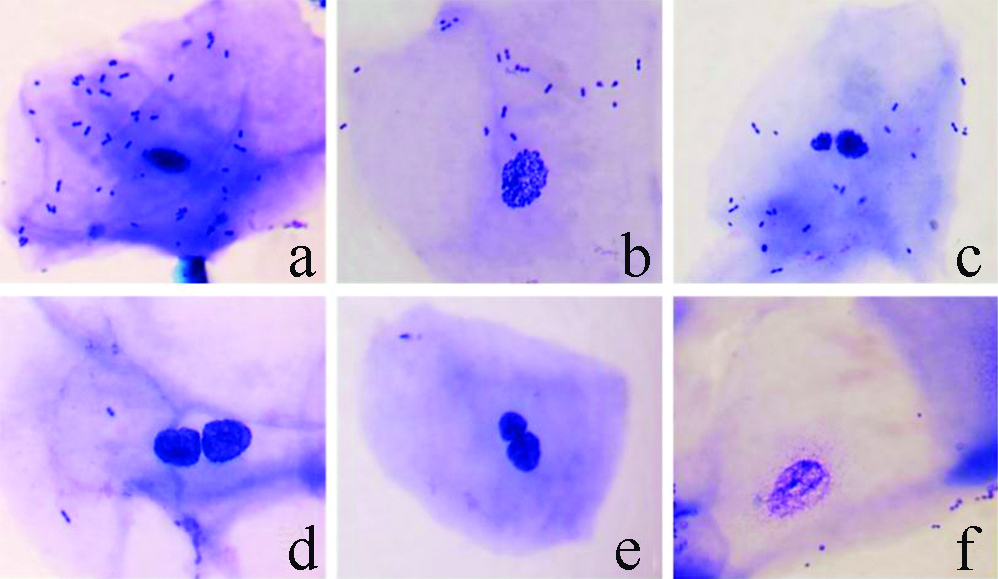
Micronucleated cells were characterised by the presence of the main nucleus and a smaller one, called micronucleus, resulting from a chromosomal fragmentation by genotoxicity. The MN cells were characterised according to the following criteria: (a) regular contour, round or elliptical, and inside the cell cytoplasm; (b) similar colour to the main nucleus; (c) less than one-third of the diameter of the nucleus; (d) completely separated from the nucleus, allowing clear identification between the nucleus and MN limits [14-17].
Pycnotic cells were characterised by a small nucleus with condensed chromatin and intense staining. Nuclear diameter is 1/3 to 2/3 smaller than that of the differentiated cells and is related to an advanced stage of cell death by necrosis. Karyorrhexis cells were characterised by more extensive chromatid aggregation indicating fragmentation and nuclear disintegration in the advanced stage of cell death by apoptosis. Binucleated cells were characterised by the presence of two nuclei with characteristics similar to differentiated cells. The presence of binucleation is indicative of failure due to cytotoxic action in the cytokinesis process during cell reproduction [17,18].
“Broken egg” cells showed the main nucleus and the nearby accessory core connected by fine chromatin filaments. The accessory core has the same morphological and colouring characteristics as the main core. However, it has a diameter less than ¼ of the core. It is believed that this type of morphology originates from the presence of dicentric chromosomes with abnormal anaphasic behaviour during segregation. Karyolytic cells have a lightly stained chromatin which is difficult to be analysed under light microscopy and related to a more advanced stage of cell death process due to necrosis [17,18].
Statistical Analysis
The data were analysed statistically using the Kruskal Wallis test at 5% significance level in SPSS version 23.0 software.
Results
The results obtained in the present study came from comparisons of the baseline data (D0) with the other collections (D14, D21 and D52, respectively). A significant increase in metanuclear alterations (p<0.0001) and the number of binucleated cells (p=0.0081) was observed between baseline D0 and D21 (last day of bleaching gel application). Regarding MN frequency, there were no significant differences between baseline and other collection times (p=0.08) [Table/Fig-3].
Median values and data distribution (first quartile-25%, and third quartile-75%) of the cellular alterations observed during each cell collection.
| Metanuclear alterations† | Binucleated cells | Micronuclei |
|---|
| Collection time | Median* | 25% | 75% | Median* | 25% | 75% | Median* | 25% | 75% |
|---|
| D0 (baseline/control) | 4.5A | 3 | 5 | 2A | 1 | 4.7 | 2A | 1 | 3 |
| D14 | 5.5AB | 4 | 9.7 | 2.5AB | 1.3 | 7.8 | 3.5A | 1.3 | 5 |
| D21 | 11.5B | 5.5 | 19 | 5.5B | 4 | 13.5 | 3A | 1.3 | 12.3 |
| D52 | 2.5A | 2 | 4.7 | 2.5A | 1 | 4 | 1A | 1 | 3.8 |
*Different letters in the same column indicate statistically significant differences by Kruskal Wallis test at 5% significance level.
†Metanuclear alterations include karyorrhexis, pycnosis, karyolysis and “broken egg”
Discussion
Tooth bleaching is a widely used treatment [5], performed with different products and substances of variable concentrations, depending on the bleaching protocol chosen. Despite the positive effect (teeth whitening) of bleaching, it may have some side effects, such as dentin sensitivity, irritation of the oral soft tissues in contact with the bleaching gel [3,19], in addition to the possibility of cell mutation [8].
For the office-bleaching technique, the most commonly used agent is 35% hydrogen peroxide which is directly applied by the dentist on the buccal surface of dental enamel. Because of the high concentration of the substance, a gingival barrier is applied before the bleaching procedure to protect oral mucosal cells. At home bleaching is often performed with lower concentrations of carbamide peroxide (10%, 16% or 22%). The agents are applied using an acetate custom tray, which is daily used by the patient for a few hours over a period of 2-3 weeks [16]. In this case, a small amount of bleaching agent can come in contact with the marginal gingiva potentially causing cells alterations [4].
Reversible cell alterations and no genotoxicity of tooth bleaching were found using 10% and 16% carbamide peroxide [4,5]. However, the genotoxicity of 22% carbamide peroxide (the highest and most effective concentration for at-home bleaching) has not been clarified by literature. Thus, the present study results can contribute to the current scientific knowledge. The method used to collect cell samples in this study was exfoliative cytology. The analysis of metanuclear alterations in exfoliated oral cells in humans is a cytogenetics technique which is minimally invasive and relatively simple and capable of monitoring DNA damage in humans [20]. Exfoliative cytology is a safe, painless procedure that can be performed during routine oral examination [21].
In vivo cells resemble closely the conditions that exist in the oral cavity during tooth bleaching [22]. Thus, the present study was designed as a clinical research, with collections based on cell turnover [4,12,13].
A previous study [23] affirms that a significant number of karyorrhexis and pycnosis could indicate cell death by necrosis or apoptosis. In this study, a significant increase in the number of metanuclear alterations was noted between baseline and third collection (D21), referring to the last day of bleaching gel application. However, these alterations decreased at D52 (31 days after the completion of treatment) to values similar to the baseline. The effects of bleaching agents are the result of the formation of free radicals that can damage a number of intracellular structures. DNA from cells exposed to chemical or physical agents can be damaged by forming fragment chromosomes, called MN, which are observed as a result of atypical mitoses. Depending on the extent of cellular damage, the consequences can include cell cycle impairment, cell death and even the formation of a neoplasm [8,22,24].
The increase of binucleation is potentially unrelated to DNA alteration and may have only occurred due to a delay in the cell division process [25]. In this study, the number of binucleated cells increased significantly between baseline (D0) and the end of treatment (D21). As with the metanuclear alterations, the number of binucleated cells returned to baseline values after 31 days (D52) of the end of treatment. Therefore, bleaching performed with 22% carbamide peroxide can cause temporary metanuclear alterations and binucleation, which are reversible in a month after the end of treatment. It is important to highlight that oxidative DNA lesions induced by bleaching agents are repaired by DNA repair systems. The base excision repair pathway is the most important cellular protection mechanism responding to oxidative DNA damage, being responsible for protecting cells and organisms from mutagenesis and carcinogenesis [8,26]. Certainly, this explains the results found in this study, in which a significant increase in binucleated cells and metanuclear alterations in D21 was noted when compared to baseline, with a decrease in changes on day 52, with values equivalent to those of baseline.
Some studies have suggested that the emergence of MN could predict the risk of cancer [8,27]. In this study, no MN frequency increase was found between baseline and other collections. Thus, at home bleaching using 22% carbamide peroxide, when performed correctly and under the dentist’s supervision in healthy patients for 21 days has not been found to be carcinogenic.
Almeida AF et al., conducted a clinical study to evaluate the genotoxic response using a micronucleus assay, after the application of two concentrations of carbamide peroxide (10% or 16%) in 37 patients [16]. No difference was observed between the two groups, at either 15 or 45 days (p=0.90). The authors concluded that when bleaching is not prolonged or not performed very frequently, bleaching agents containing carbamide peroxide alone will not cause mutagenic stress on gingival epithelial cells. Despite having analysed carbamide peroxide in lower concentrations, the results obtained by Almeida AF et al., are comparable with the present study, in which only transient metanuclear alterations were identified after home bleaching treatment [16].
Monteiro MJF et al., conducted a clinical study evaluating the genotoxic potential of 10% hydrogen peroxide in patients to at-home bleaching [28]. The micronucleus count did not indicate genotoxic potential 10% hydrogen peroxide. The authors concluded, similar to the present study, that no genotoxic effects were observed in patients submitted to at-home bleaching systems (30 min/day for 14 days), even 30 days after the end of treatment.
De Toni AR et al., evaluated through the MN test the genetic damage to the normal gingival mucosa exposed to 15% carbamide peroxide gel in at-home bleaching procedure in forty five patients [29]. Significant difference was noted when comparing the collections of day 0 and day 24 of the experimental group (p=0.002). They concluded that carbamide peroxide showed genotoxic activity in vivo. Such results differ from the present study, since we didn’t observe variation in the number of MN in the different cell collections performed. It is also noteworthy that in the present study the last analysis was carried out 31 days after the end of the bleaching, whereas De ToniA R et al., performed their last analysis just 10 days after the completion of the bleaching, which may have overestimated their final results [29].
In view of the varying results obtained by different studies regarding the genotoxicity of bleaching agents, the effect of these substances on oral mucosa cells would be important to be evaluated in future investigations.
In this study, although there were less number of participants, the sample selection was similar to previous studies in which only healthy patients without any previous exposure to mutagenic compounds and within the age group determined were selected [22,23]. Volunteers aging from 20 to 30 years were selected in order to standardise the sample and because this is the age group that most seeks dental bleaching treatment [22].
Limitation(s)
The sample size of the study population was relatively small. Follow-up data was not collected after the last cell collection-performed 31 days after the end of bleaching.
Conclusion(s)
Although at-home bleaching using 22% carbamide peroxide can cause temporary effects on mucosal cells, the alterations disappear one month after finishing the bleaching treatment. Therefore, this bleaching protocol does not have permanent genotoxicity effect on oral cells. Thus, based on the results of this research, patients should not be discouraged from performing home tooth bleaching.
*Different letters in the same column indicate statistically significant differences by Kruskal Wallis test at 5% significance level.
†Metanuclear alterations include karyorrhexis, pycnosis, karyolysis and “broken egg”