Periodontitis is an inflammatory disease caused by microorganisms that colonise the gingival sulcus [1]. An early step of any inflammatory response to microbial infection is recruitment of Polymorphonuclear Neutrophils (PMNs) which migrate into lamina propria from the microvasculature at the site of infection [2]. Transepithelial migration of PMNs is the hallmark of inflammation and has an important role in the clearance of mucosal infections [3,4]. As with any other infectious disease, neutrophils are recruited early in periodontal disease and accumulate within the gingival connective tissue, the junctional epithelium and migrate into gingival crevice [5]. The migration of neutrophils into gingival crevice is the first line of defence against the subgingival plaque and form a barrier that prevents the subgingival microbes to invade the deeper tissues [6,7]. This is the prime reason why severe periodontitis is seen in patients with a lack of neutrophils or with neutrophil defects [8,9]. Although neutrophils are mandatory for maintaining health of the periodontium, their excess also seems to be detrimental. Excess of neutrophils within the periodontal tissues leads to tissue destruction [10]. Thus, both lack and excess of neutrophils lead to inflammation mediated bone loss and tissue destruction in periodontitis. This bone loss is then evident as pocket formation and CAL in the patients [11].
It is estimated that there are around 30,000 neutrophils constantly in transit through periodontal tissues every minute [12]. Oral rinse from subjects with healthy gingiva and those with periodontitis contain significant number of neutrophils [13]. This ONC is also termed as Orogranulocytic Inflammatory Load (OIL). The OIL increases as the inflammation of periodontium increases [14]. The concept of using ONC to assess periodontal disease status was introduced by Raeste AM et al., in 1978 [15]. Several methods have been suggested thereafter to determine oral inflammatory load, all of which quantify oral neutrophils in an oral rinse. The methodology used to assess the count of neutrophils varies considerably and is difficult to perform in routine clinical setting [13,14,16].
Periodontal disease is traditionally diagnosed with assessment of damage to periodontium through measurement of probing depth and CAL [17]. Periodontal disease was originally considered to be slowly progressive with continuous bone loss. Several researches conducted for disease activity found that periodontal disease progresses through periods of exacerbation and remission [18,19]. Several clinical, microbiological, radiographic and host response parameters have been researched in attempt to develop a diagnostic test that identifies the risk of development of active disease [20,21]. Many of these tests appear promising but the cost and methodology involved prevents their widespread application. Thus, the primary aim of this study was to assess whether an economical, easily available methylene blue dye and simple chair side technique can be used to assess oral inflammatory load rather than previously described cumbersome and expensive laboratory techniques. Further, this simple method was utilised to assess if presence of periodontal disease and its severity can increase oral inflammatory load.
Materials and Methods
A case control study was designed to assess whether the periodontal disease increases the OIL by assessing the migration of oral neutrophils into the oral cavity in Periodontitis patients and PH subjects. The study was carried out in the Department of Dentistry, ESIC Medical College and Hospital, Faridabad from September 2017 till April 2018.
Subjects for the study were in the age group of 18-60 years, from both sexes and were recruited from the outpatient department of the hospital. The study protocol was explained to each subject and a written informed consent was obtained prior to inclusion into the study. The study was approved by the Institutional Ethics Review Committee (134/A/11/16/Academics/MC/2016/109-A).
The study was designed based on a pilot study conducted prior to calculating the sample size. Based on these calculations, fifty subjects were enrolled in two different groups: CP and PH. Disease group patients had severe periodontitis (13 males and 12 females, mean age 34.08±5.139 years). Subjects in CP group had more than 10 pockets that were more than 5mm in depth and attachment loss around more than 30% of sites with CAL ≥5 mm around the involved teeth [22]. The control group (11 males and 14 females mean age 32.52±6.875 years) had no clinical signs of periodontal disease.
A medical history was taken to ensure that all participants were in good systemic health. Patients with diseases that could affect immune function/neutrophil response such as haematological disorders or diabetes were excluded. Patients with oral pathologies other than periodontitis such as apthous ulcers or tonsillitis which could alter the OIL were not included. Subjects who underwent any form of periodontal therapy, tobacco users, and pregnant females were also excluded.
A periodontal examination was performed on all the patients on all teeth present except third molars. Pocket PD, CAL and BOP were assessed on six sites per tooth (mesiobuccal, mid buccal, distobuccal, mesio lingual, mid lingual and distolingual). All the measurements were made using a UNC-15 periodontal probe (HU-Friedy). Percentage of sites with positive BOP, Average PD and CAL were calculated for each patient. While PD and CAL were taken as a measure of disease severity, percentage sites with BOP (no of sites with BOP/total sites x 100) were taken as a measure of disease activity.
Collection of Salivary Samples and Isolation of Oral Neutrophils: Prior to conducting the clinical examination, the subjects were asked to rinse with 10ml of normal saline (0.9% sodium chloride (Albert David Ltd., Ghaziabad) for 30 seconds. The saliva expectorate was then collected into a 50ml sterile plastic container. The same procedure was carried out to obtain a subsequent rinse after 10 minutes. The purpose of taking two samples 10 minutes apart was to check the reproducibility of the sample in the same patient. All samples were processed within two hours of collection of sample.
The rinse samples were processed at 3000 rpm for 5 minutes in a centrifuge (R-85, Remi sales and engineering Ltd., Mumbai) to extract the supernatant. The isolated supernatant was then re-suspended in 5 mL of Hank’s Balanced salt solution (Sigma-Aldrich, India). Methylene Blue Dye (1% aqueous solution, RNTPC, Faridabad) was added to 1mL of this solution to stain the oral neutrophils.
A 10 μL aliquot of this suspension was assessed on a haemacytometer (Bright-Line™ Hemacytometer, Sigma Aldrich, India) and neutrophils were counted under 40x magnification on a light microscope. Both the rinses taken ten minutes apart were assessed and compared for any differences.
Statistical Analysis
Means with standard deviations was calculated for all the demographic (age and sex ratio) and clinical characteristics. Log transformation was used to normalise the distribution before statistical analysis of oral neutrophils, where in 106 was represented as base (0) on log scale. To compare the demographic and clinical characteristics of the two groups unpaired t-test was applied. To compare the statistical difference between the two rinses in same subject, a paired t-test was utilised. Since there was a non-normal distribution of data for clinical parameters, Spearman’s correlation coefficient was applied to assess the association between log transformed neutrophils and clinical parameters. All statistical tests were carried out using suitable software (online social science statistics calculator) and a value of p≤0.05 was considered significant.
Results
Both the groups: CP and PH consisted of comparable numbers of males and females. There was no significant difference in the mean age of both the groups (p>0.05). Mean values of clinical parameters (% sites with BOP, PD, CAL) and ONC counts are summarised in [Table/Fig-1]. All the clinical parameters were significantly increased in CP Group compared to PH group (p<0.001). The two rinses taken ten minutes apart showed no significant difference in ONC (p>0.05). On comparing, ONC count was significantly higher in the CP group compared to PH group (p<0.001).
Comparison of mean clinical parameters and mean ONC of study groups.
| Clinical parameters | Periodontally Healthy (PH) (Mean±Standard deviation) | Chronic Periodontitis (CP) (Mean±Standard deviation) | t-value# | p-value |
|---|
| Percentage sites with BOP (%) | 1.24±0.969 | 74.96±11.069 | -33.171 | <0.001* |
| Probing depth (mm) | 1.507±0.437 | 4.508±0.905 | -14.918 | <0.001* |
| Clinical attachment loss (mm) | 0 | 4.46±1.192 | -18.697 | <0.001* |
| Oral neutrophil counts (nx106) | 0.454±0.066 | 3.644±1.096 | -14.525 | <0.001* |
#t-test; *<0.001 (highly significant)
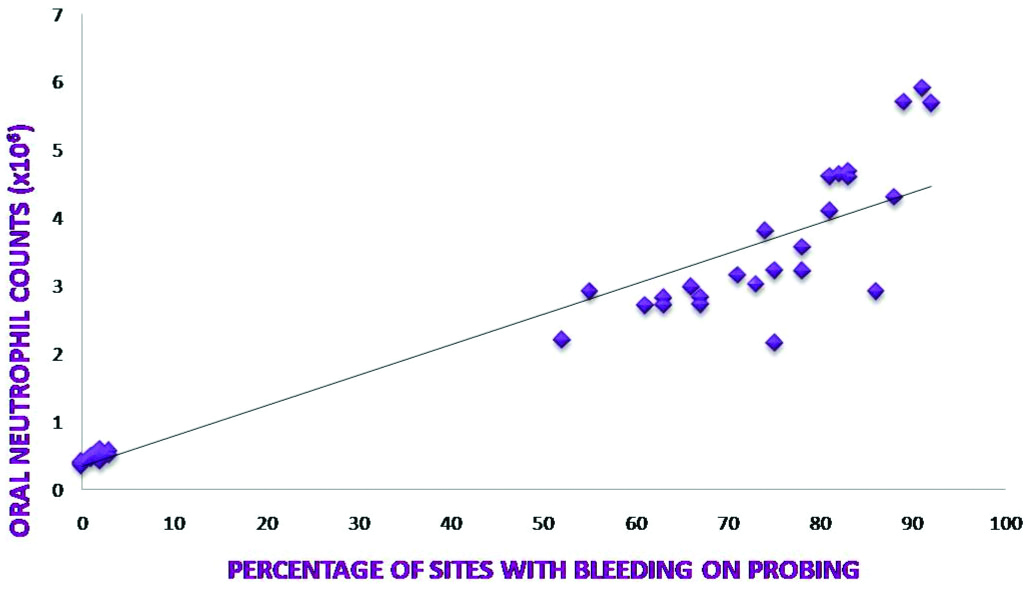
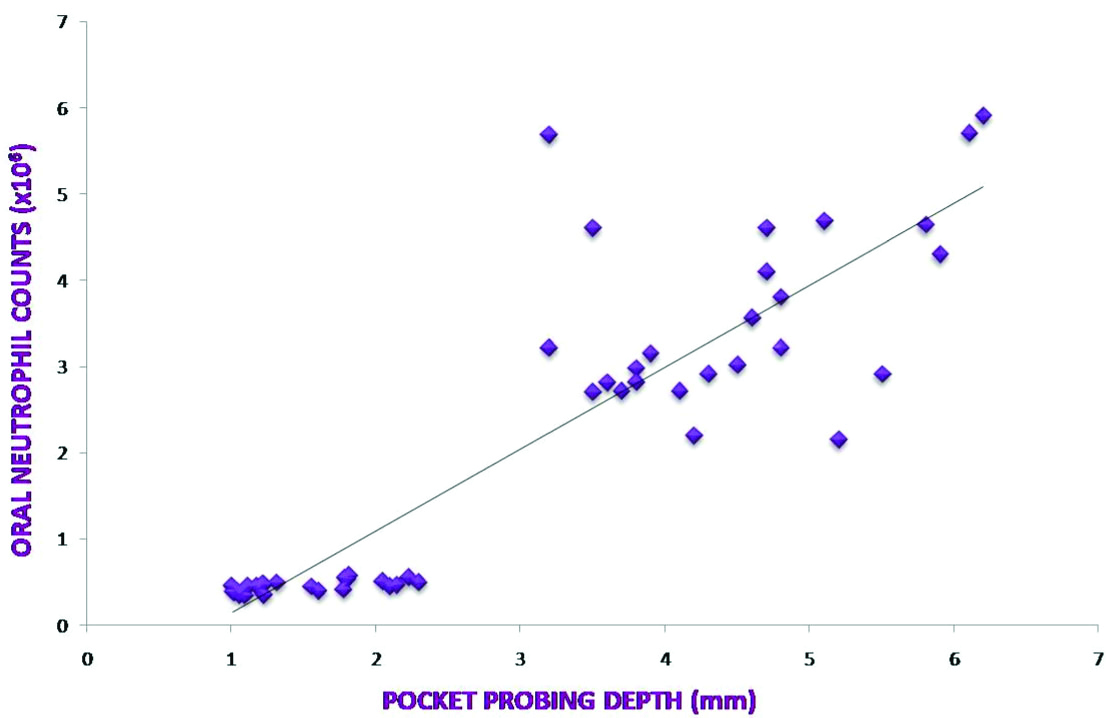
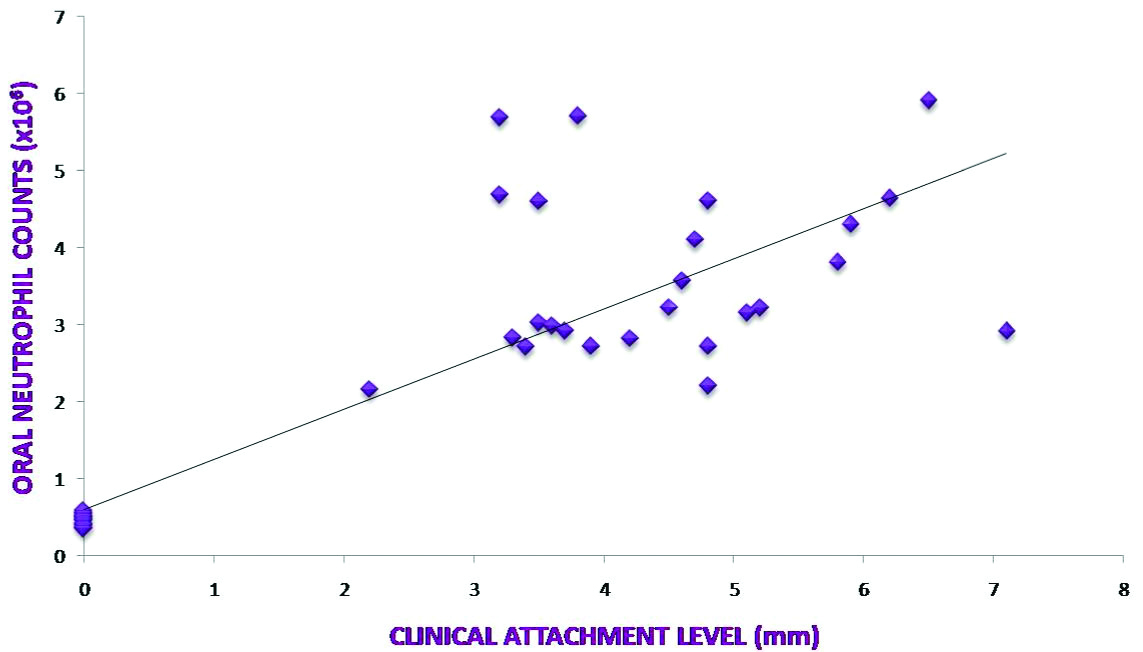
A relationship between clinical parameters and ONC was assessed to find out if any co-relation exists between ONC and severity and activity of periodontal disease [Table/Fig-2]. Spearman’s co-relation showed a highly significant, positive correlation between ONC and all clinical parameters (p<0.001) [Table/Fig-3,4 and 5].
Co-relation between clinical parameters and ONC.
| r-value† | p-value |
|---|
| Percentage sites with BOP and ONC | 0.95766 | <0.001‡ |
| Mean probing depth and ONC | 0.87699 | <0.001‡ |
| Mean clinical attachment loss and ONC | 0.82811 | <0.001‡ |
†Spearman’s correlation coefficient; ‡ <0.001 Highly significant
Co-relation between Percentage sites with BOP and ONC.
Co-relation between Mean Probing Depth and ONC.
Co-relation between Mean Clinical Attachment Level and ONC.
Discussion
Histological evidence has suggested that neutrophils form a wall between the junctional epithelium and subgingival dental plaque. Thus, even in a healthy periodontal state, there is a constant migration of neutrophils, hence the presence of OIL in healthy subjects [13].
The primary objective of the study was to develop a simple, reproducible oral rinse assay that easily determines ONC to quantify OIL. The test suggested by the authors is a non-invasive test that is not technique-sensitive and can be easily applied to assess OIL. To the best of our knowledge all other techniques previously tested for OIL consist of cumbersome methodology or use of expensive equipment which makes the quantification non practical in clinical setting. While one of the techniques used acridine orange as a dye and a fluoroscence microscope to count neurophils, another study used 2,2’-azino-bis (3-ethylbenzo-thiazoline-6-sulfonic acid) (ABTS) as dye and automated microplate reader to count the neurophils. Both these techniques require costly and cumbersome laboratory materials and equipment [14,16].
BOP illustrates clinical, histological and microbiological alterations associated with periodontal disease [23]. Additionally, clinical and histologic data advocate that bleeding is an earlier sign of gingivitis than other visual signs of inflammation (redness and swelling). It has been evaluated for its prognostic value in identifying sites at risk for periodontal breakdown during the maintenance phase of periodontal therapy [24].
The results from our study indicate a very strong positive correlation between BOP and ONC. Earlier studies based on cumbersome techniques also revealed similar correlations. It has been already demonstrated that the rate of migration of oral neutrophils positively correlated with gingival index [25]. Therefore, increased OIL can be taken as an indicator of increased inflammation which in turn indicates disease activity.
Over the years, the severity of periodontal disease has been defined by depth of periodontal pocket and/or CAL. The present data indicate a strong positive correlation between ONC and increased probing depth as well as CAL. This positive association is likely as increased pocket depth lead to an increased ulcerated epithelium through which an increased oral neutrophil migration could occur leading to increased counts. Previous studies also indicate a highly positive correlation between ONC in saliva and severity of periodontal disease [13,14]. This directs to a conclusion that OIL could be used as a marker for severity of periodontal disease.
In a recent work, Huda S et al., suggested that if inflammatory activity associated with periodontitis could be measured accurately, it might be possible to correlate OIL to adverse pregnancy outcomes [16]. Their results too advocated the use of rinse assay as a screening tool to assess the presence of periodontitis in pregnant females.
The use of clinical parameters to assess periodontal disease seems to be flawed in certain scenarios. Many dental professionals focus on gingival bleeding, especially its presence or absence during periodontal probing, as an indicator of active periodontal disease. However bleeding upon probing alone is unreliable as a disease indicator. Similarly, the mere presence of pocket depth does not indicate presence of disease. Chronic periodontal disease proceeds through a series of random episodic bursts. Periodontal sites can exist as either disease active or disease inactive sites. During periods of disease activity, these sites show evidence of inflammation either clinically or histologically with a corresponding increase in probing depths, whereas during the inactive state, no significant change in probing depth and inflammatory change can be detected [26]. A deep pocket that has not shown an evidence of disease cannot be termed as an indicator of active disease [27].
Similarly, CAL happening as a consequence of tooth brush trauma which shows no on-going periodontal breakdown cannot be used to assess severity of disease. OIL on the other hand can be a key indicator to identify active disease from quiescent. OIL is bound to increase only in presence of active inflammation of the pocket epithelium and would offer a better indicator of disease activity and severity. Interestingly, one of the very first oral rinse studies to assess oral neutrophil counts also correlated ONC with severity of periodontal disease [14]. They found a threefold increase in ONC in CP and 25 fold increase in acute periodontitis cases. Therefore, current research suggests that, ONC or OIL can be taken as a predictor of current status of periodontal disease.
Limitation(s)
One of the limitations of this study is that this is not a comparative study where methylene blue dye was compared with previously used dyes. A comparative study would have better established the efficacy of methylene blue.
Conclusion(s)
Our results indicate that ONC which quantify the OIL, correlate positively with periodontal disease activity and severity. The use of this chair side technique may help in easy screening of periodontal disease status in a dental set up as it offers advantages of being easy and non technique-sensitive.
#t-test; *<0.001 (highly significant)
†Spearman’s correlation coefficient; ‡ <0.001 Highly significant