Haemorrhagic body fluids samples are commonly received for cytological evaluation [1]. The presence of erythrocytes partially or completely obscures the morphological details of representative cells and thus poses great diagnostic difficulty [2]. Haemorrhagic body fluids are processed by using various techniques with a common goal of concentrating adequate number of cells having intact cellular morphology, without losing the diagnostic cells during processing. Most commonly used reagents and methods are CF, Glacial Acetic Acid (GAA), NSRT, saponin method, cellular fixation and concentration methods [1].
Air drying has pernicious effects on the nuclear and cytoplasmic staining characteristics [3]. Hence rehydration techniques have been applied in processing haemorrhagic effusions [4,5] and Fine Needle Aspiration Cytology (FNAC) smears. The rehydration of dry samples offers great advantages over the conventional wet fixation [3,6]. However, nuclear shrinkage and cytoplasmic swelling has been documented in few studies [1]. To the best of the knowledge of the authors, the lemon solution has not been used as a rehydrating solution to process haemorrhagic cytology samples in the past. The present study highlights the utility of LSRT as a novel rehydration technique developed by using 10% lemon solution for processing haemorrhagic cytology samples.
The present study was undertaken to process the haemorrhagic samples by using LSRT as a new technique and compare three different haemolysing solutions, namely normal saline by NSRT, CF, and 10% lemon solution by LSRT.
Materials and Methods
The present analytical study was conducted at cytopathology section in the department of Pathology at a rural tertiary care institute, PESIMSR, Kuppam, Andhra Pradesh, India. It was a prospective study over a period of two months from June 2016 to July 2016. Ethical clearance was obtained from the institutional ethical committee bearing number PESIMSR/IHEC/08. The data was collected with the help of pro forma which included variables like particulars of the patient, clinical diagnosis and cytomorphological details. The sample size was calculated using the following formula:
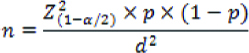
“n” is the sample size
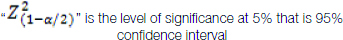
“p” is the expected proportion of haemorrhagic cytology samples
“d” is the desired error of margin
All cases in which cytology samples (cytology fluid samples and FNAC samples) were haemorrhagic were included in the study. Those cases in which specimen were inadequate for evaluation by different techniques were excluded from the study. The haemorrhagic cytology samples were processed as depicted in the flowchart [Table/Fig-1].
Flow chart depicting the processing of hemorrhagic cytology samples.
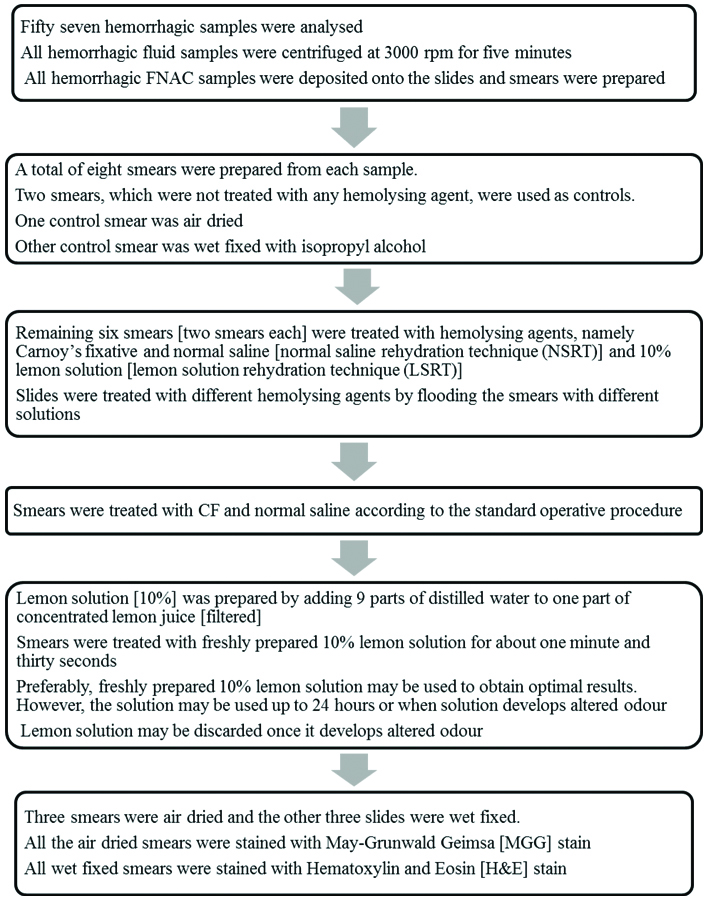
Haemolysis in smear background, retention of epithelial cells or mesothelial cells or inflammatory cells and cytomorphological details were documented in these three types of smears prepared after treating with haemolysing solutions (CF, NSRT and LSRT) and compared with the control smears. All smears were evaluated by single pathologist by using a scoring system.
Scoring System
Smear scoring was done according to the modified scoring system as given by Ng WF et al., [4]. Number of erythrocytes in smear background was scored as score 1 (same as control), score 2 (approximately, 75% of the control), score 3 (approximately, 50% of the control), score 4 (approximately, 25% of the control). Retention of epithelial/mesothelial cells/inflammatory cells was scored as score 1 (approximately, 25% of the control), score 2 (approximately, 50% of the control), score 3 (approximately, 75% of the control), score 4 (same as that of control). Cytomorphological details such as nuclear features (chromatin and shrinkage), cytoplasm staining, cell borders and back ground material in the smears were assessed. Cytomorphological details was scored as 1 (very poor- unsuitable for assessment), score 2 (sub-optimal- just acceptable for assessment), score 3 (optimal with nuclear and cytological features) and score 4 (excellent preservation and sharp nuclear and cytological features).
Statistical Analysis
Descriptive statistics with percentages were analysed. Chi-square test and Fischer-exact test were applied for statistical difference in values. All results were analysed by considering statistical significance at a level of p-value <0.05. The statistical analysis was done using statistical software STATA version 13.
Results
During the study period, 60 haemorrhagic cytology samples were received. Three cases were excluded from the study. All the three cases were inadequate samples from FNAC of thyroid. Fifty seven cases were analysed. Out of 57 cases of haemorrhagic cytology samples, FNAC samples constituted the majority in comparison with haemorrhagic body fluid samples. Among the FNAC samples, majority of cases were aspirates from thyroid. Among haemorrhagic body fluid samples, majority were of pleural fluid [Table/Fig-2].
Distribution of spectrum of cytopathology diagnoses.
| Sl. No. | Cytopathology diagnoses | Cases (n=57) | Percent (%) |
|---|
| 1 | FNAC samples | 41 | 71.93 |
| Thyroid | 36 | 63.16 |
| Chronic lymphocytic thyroiditis- Bethesda category II | 15 | 26.32 |
| Nodular hyperplasia-Bethesda category II | 11 | 19.3 |
| Nodular colloid goiter- Bethesda category II | 4 | 7.02 |
| Benign thyroid lesion- Bethesda category II | 4 | 7.02 |
| Benign cystic lesion- Bethesda category I | 1 | 1.75 |
| Hurthle cell neoplasm-Bethesda category IV | 1 | 1.75 |
| Right breast | 2 | 3.51 |
| Fibroadenoma | 1 | 1.75 |
| Invasive ductal carcinoma- intermediate differentiated | 1 | 1.75 |
| Left breast | 2 | 3.51 |
| Proliferative breast disease with atypia | 1 | 1.75 |
| Invasive ductal carcinoma- intermediate differentiated | 1 | 1.75 |
| Cervical lymph node | 1 | 1.75 |
| Metastatic carcinoma -poorly differentiated carcinoma | 1 | 1.75 |
| 2 | Body fluid samples | 16 | 28.07 |
| Pleural fluid | 6 | 10.53 |
| Negative for malignancy (Reactive effusion) | 6 | 10.53 |
| Ascitic fluid | 4 | 7.02 |
| Negative for malignancy | 4 | 7.02 |
| Peritoneal washings | 3 | 5.26 |
| Negative for malignancy | 2 | 3.51 |
| Positive for malignancy (adenocarcinoma) | 1 | 1.75 |
| Synovial fluid | 2 | 3.51 |
| Negative for malignancy | 2 | 3.51 |
| Cerebrospinal fluid | 1 | 1.75 |
| Negative for malignancy | 1 | 1.75 |
In the present study, the patients’ age ranged from 18 years to 75 years (mean=39.73 years). Females were affected in majority of cases {46 Cases (80.70%)} with M:F ratio of 1:4.2.
Cytopathology Diagnoses
Among FNAC cases, thyroid swellings were most frequent aspirated lesions. Most common cytologic diagnostic entity was chronic lymphocytic thyroiditis. Cervical lymph node was the least common site of aspiration and was diagnosed as metastatic carcinoma-poorly differentiated. The distribution of spectrum of lesions is depicted in [Table/Fig-2].
Among haemorrhagic body fluid samples, pleural fluid was the most common haemorrhagic sample. All six cases were negative for malignancy. Cerebrospinal Fluid (CSF) was least common haemorrhagic sample and was negative for malignancy [Table/Fig-2].
Effect of Haemolysing Solutions on Smear Background
MGG stained smears
Best haemolysing effect was seen with NSRT, followed by LSRT and CF. The p-value was highly significant [Table/Fig-3].
Effect of haemolysing solutions on smear background, Retention of cells and cytomorphological details in MGG stained smears.
| Effect of haemolysing solutions on smear background in MGG stained smears |
|---|
| Processing method | Number of cases | Average score | p-value |
|---|
| Score 1 | Score 2 | Score 3 | Score 4 | Total cases |
|---|
| CF | 0 (0%) | 11 (19.30%) | 32 (56.14%) | 14 (24.56%) | 57 | 3.05 | p<0.0001* |
| NSRT | 0 (0%) | 1 (1.75%) | 11 (19.30%) | 45 (78.95%) | 57 | 3.77 |
| LSRT | 0 (0%) | 1 (1.75%) | 12 (21.05%) | 44 (77.19%) | 57 | 3.75 |
| Effect of haemolysing solutions on Retention of epithelial/ mesothelial cells/inflammatory cells in MGG stained smears |
| Processing method | Number of cases | Average score | p-value |
| Score 1 | Score 2 | Score 3 | Score 4 | Total cases |
| CF | 7 (12.28%) | 4 (7.02%) | 4 (7.02%) | 42 (73.68%) | 57 | 3.42 | p=0.035* |
| NSRT | 5 (8.77%) | 9 (15.79%) | 8 (14.04%) | 35 (61.40%) | 57 | 3.28 |
| LSRT | 2 (3.51%) | 1 (1.75%) | 5 (8.77%) | 49 (85.96%) | 57 | 3.77 |
| Effect of haemolysing solutions on cytomorphological details in MGG stained smears |
| Processing method | Number of cases | Average score | p-value |
| Score 1 | Score 2 | Score 3 | Score 4 | Total cases |
| CF | 0 (0%) | 0 (0%) | 50 (87.72%) | 7 (12.28%) | 57 | 3.12 | p<0.0001* |
| NSRT | 5 (8.77%) | 11 (19.30%) | 39 (68.42%) | 2 (3.51%) | 57 | 2.66 |
| LSRT | 0 (0%) | 4 (7.02%) | 49 (85.96%) | 4 (7.02%) | 57 | 3.00 |
*Chi-square test was the statistical tool used to perform statistical analysis
H&E stained smears
Best haemolytic effect was seen with LSRT, followed by NSRT and CF. The p-value was highly significant [Table/Fig-4].
Effect of haemolysing solutions on smear background, retention of cells and cytomorphological details in H&E stained smears.
| Effect of haemolysing solutions on smear background in H&E stained smears |
|---|
| Processing method | Number of cases | Average score | p-value |
|---|
| Score 1 | Score 2 | Score 3 | Score 4 | Total cases |
|---|
| CF | 0 (0%) | 25 (43.86%) | 24 (42.11%) | 8 (14.04%) | 57 | 2.70 | p<0.0001* |
| NSRT | 0 (0%) | 8 (14.04%) | 25 (43.86%) | 24 (42.11%) | 57 | 3.28 |
| LSRT | 0 (0%) | 4 (7.02%) | 21 (36.84%) | 32 (56.14%) | 57 | 3.49 |
| Effect of haemolysing solutions on retention of epithelial/ mesothelial cells/ inflammatory cells in H&E stained smears |
| Processing method | Number of cases | Average score | p-value |
| Score 1 | Score 2 | Score 3 | Score 4 | Total cases |
| CF | 2 (3.51) | 9 (15.79%) | 3 (5.26%) | 43 (75.44%) | 57 | 3.52 | p=0.029* |
| NSRT | 6 (10.53%) | 7 (12.28%) | 8 (14.04%) | 36 (63.16%) | 57 | 3.29 |
| LSRT | 1 (1.75%) | 2 (3.51%) | 4 (7.02%) | 50 (87.72%) | 57 | 3.80 |
| Effect of haemolysing solutions on cytomorphological details in H&E stained smears |
| Processing method | Number of cases | Average score | p-value |
| Score 1 | Score 2 | Score 3 | Score 4 | Total cases |
| CF | 0 (0%) | 1 (1.75%) | 51 (89.47%) | 5 (8.77%) | 57 | 3.07 | p=0.281* |
| NSRT | 1 (1.75%) | 0 (0%) | 55 (96.49%) | 1 (1.75%) | 57 | 2.98 |
| LSRT | 0 (0%) | 0 (0%) | 55 (96.49%) | 2 (3.51%) | 57 | 3.03 |
*Chi-square test was the statistical tool used to perform statistical analysis
In all the three processing techniques, haemolysing effect of haemolysing solutions was better in MGG smears than H&E smears. The p-value was statistically significant (p=0.016).
In all the three processing techniques, the average scores for haemolysing effect of haemolysing solutions were better in MGG smears than H&E smears. In all the three processing techniques, the average haemolysis percent were better in MGG smears than H&E smears [Table/Fig-5].
Comparison of average scores of haemolysing solutions, average haemolysis percent and average cell retention percent of haemolysing solutions.
| Comparison of average scores of haemolysing solutions |
|---|
| Processing method | Haemolysis (average score) | Retention of epithelial/ mesothelial cells/inflammatory cells (average score) | Cytomorphological details (average score) | Total score |
|---|
| MGG | H&E | MGG | H&E | MGG | H&E | MGG | H&E |
| CF | 3.05 | 2.70 | 3.42 | 3.52 | 3.12 | 3.07 | 9.59 | 9.29 |
| NSRT | 3.77 | 3.28 | 3.28 | 3.29 | 2.66 | 2.98 | 9.71 | 9.55 |
| LSRT | 3.75 | 3.49 | 3.77 | 3.80 | 3.00 | 3.03 | 10.52 | 10.32 |
| Comparison of average haemolysis percent and average cell retention percent of haemolysing agents |
| Processing method | Haemolysis (average score) | Retention of epithelial/mesothelial cells/inflammatory cells (average score) |
| MGG | H&E | MGG | H&E |
| CF | 54.38 | 46.32 | 82.80 | 87.72 |
| NSRT | 73.85 | 63.16 | 79.82 | 81.05 |
| LSRT | 73.68 | 64.16 | 93.33 | 94.74 |
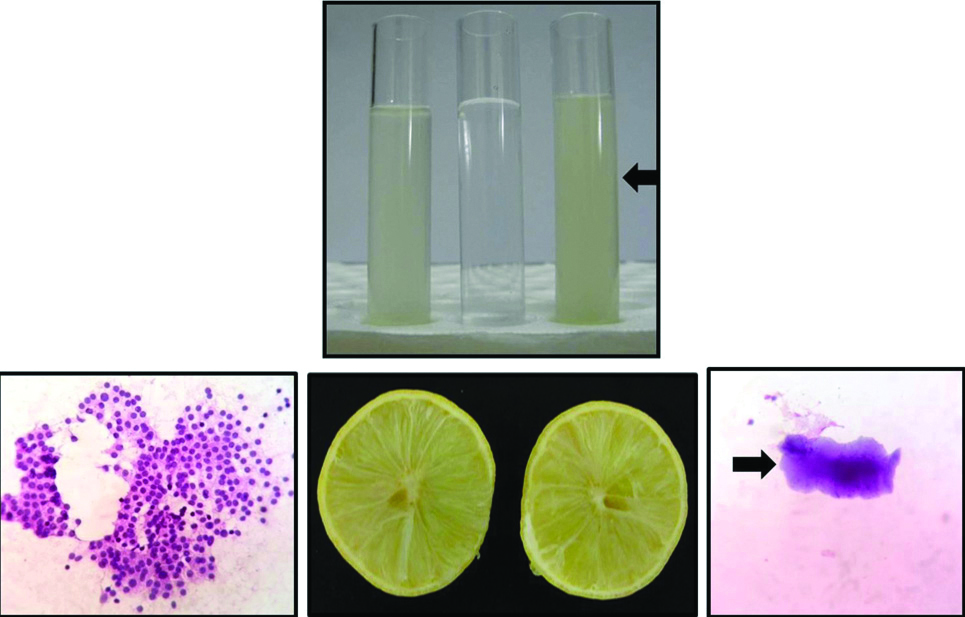
NSRT showed clear background in haemolysed areas in majority of cases in both H&E stained smears {52 cases (91.22%)} and MGG smears {54 cases (94.73%)}. CF did not show clear background, but showed pinkish material in majority of cases in both H&E smears {50 cases (87.72%)} and MGG smears {48 cases (84.21%)}. LSRT showed occasional fruit pulp artefacts in the background in few cases in both H&E smears {6 cases (10.53%)} and MGG smears {5 cases (8.77%)}, but did not interfere with interpretation of the smear or diagnosis of the lesion [Table/Fig-6].
Comparison of properly filtered 10% lemon solution (left test tube), distilled water (middle test tube) and improperly filtered 10% lemon solution (Right test tube) with fruit pulp artefact (black arrow).
Effect of Haemolysing Solutions on Retention of Epithelial Cells/Mesothelial Cells/Inflammatory Cells
MGG stained smears
Maximum retention was seen with LSRT, followed by CF and NSRT. The p-value was significant [Table/Fig-3].
H&E stained smears
Maximum retention was seen with LSRT, followed by CF and NSRT. The p-value was significant [Table/Fig-4].
In all the three processing techniques, the average scores for cell retention were better in H&E smears than MGG smears. In all the three processing techniques, the average cell retention percent were better in H&E smears than MGG smears [Table/Fig-5].
Effect of Haemolysing Solutions on Cytomorphological Details in H&E Stained Smears
MGG stained smears
Best cytomorphological details were seen with CF, followed by LSRT and NSRT. The p-value was highly significant [Table/Fig-3].
H&E stained smears
Best cytomorphological details were seen with CF, followed by LSRT and NSRT. The p-value was not significant [Table/Fig-4].
For CF processing technique, the average scores for cytomorphological details were better in MGG smears than H&E smears. For LSRT and NSRT, the average scores for cytomorphological details were better in H&E smears than MGG smears [Table/Fig-5].
Nuclear shrinkage, cytoplasmic shrinkage and minimal loss of chromatin material was commonly observed in H&E smears. Blurring of nuclear margin and loss of cytoplasmic border was observed in MGG smears.
Nuclear shrinkage and cytoplasmic shrinkage {7 cases (12.28%) H&E and 3 cases (5.26%) MGG}, Minimal loss of chromatin material {10 cases (17.54%) H&E and 3 cases (5.26%) MGG}, Blurring of nuclear margin {4 cases (7.02%) H&E and 11 cases (19.3%) MGG} and Loss of cytoplasmic border {15 cases (26.32%) H&E and 20 cases (35.08%) MGG} were seen commonly in NSRT. Neutrophils were relatively more susceptible to morphological alterations when compared to lymphocytes, macrophages, epithelial cells and mesothelial cells.
[Table/Fig-7,8,9 and 10] are microphotographs showing the effect of haemolysis solutions in MGG stained section in a case of chronic lymphocytic thyroiditis (Case 2) in FNAC smears. [Table/Fig-11,12,13 and 14] are microphotographs effect of haemolysis solutions in H&E smears in a case of gastric adenocarcinoma (Case 32) in peritoneal washings fluid cytology.
Microphotograph of FNAC smear of Chronic lymphocytic thyroiditis (Case 2) showing Hurthle cell (Thick black arrow) cluster, follicular cell cluster (Red arrow) admixed with lymphocytes. Background shows numerous erythrocytes (Notched thin black arrow). (Control slide, MGG, X400).
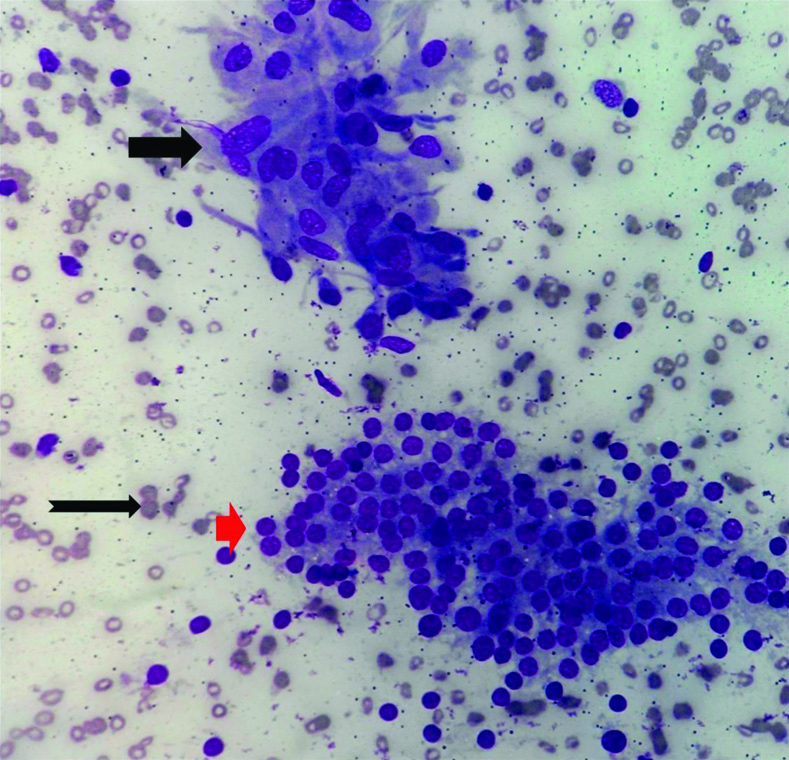
Microphotograph of FNAC smear of chronic lymphocytic thyroiditis (Case 2) showing follicular cell cluster (Small black arrow) admixed with lymphocytes. Background shows few erythrocytes. (CF, MGG, X400).
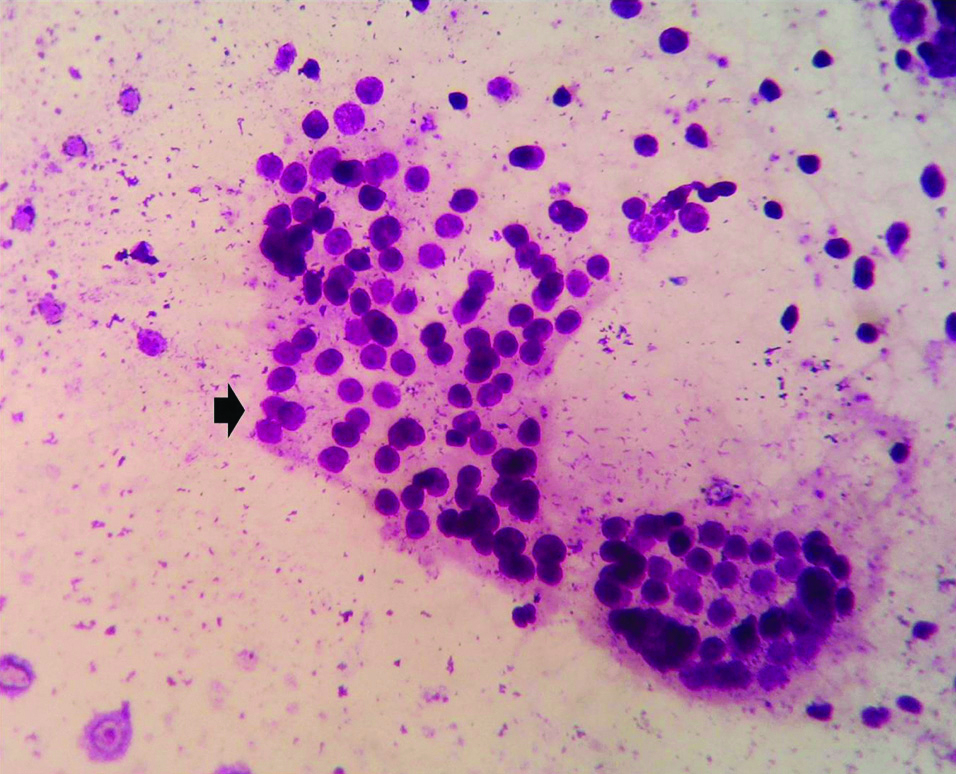
Microphotograph of FNAC smear of chronic lymphocytic thyroiditis (Case 2) showing follicular cell cluster (Thick black arrow) and admixed lymphocytes against a clear background. (NSRT, MGG, X400).
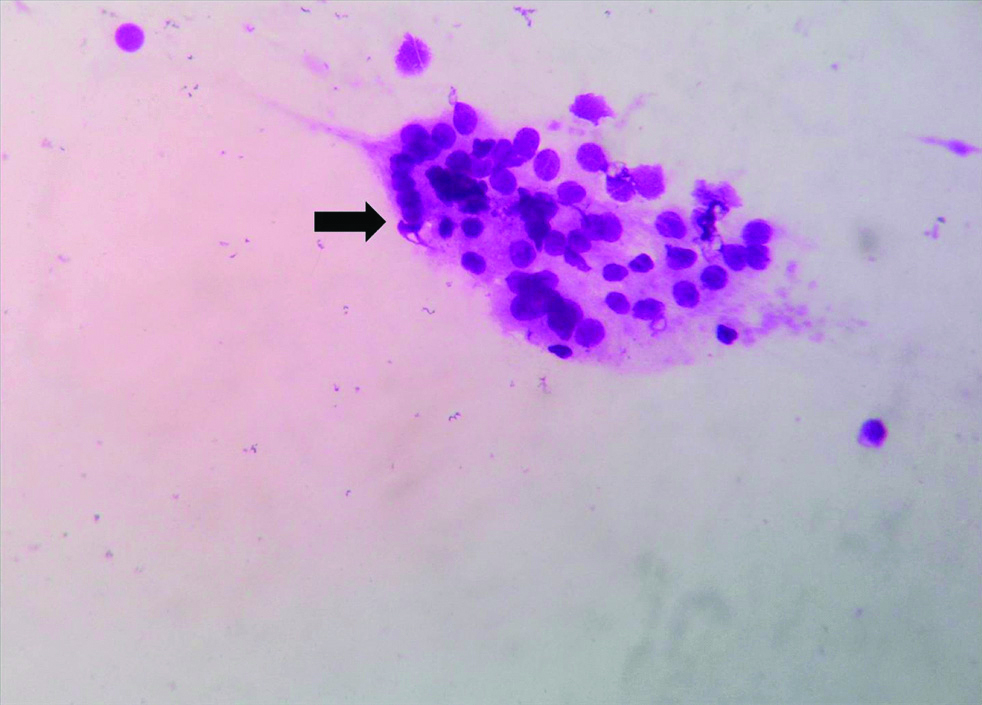
Microphotograph of FNAC smear of chronic lymphocytic thyroiditis (Case 2) showing Hurthle cells (Thick black arrow) cluster, plasma cells (Red arrow) and lymphoid cells (Notched thin black arrow) against a clear background. (LSRT, MGG, X400).
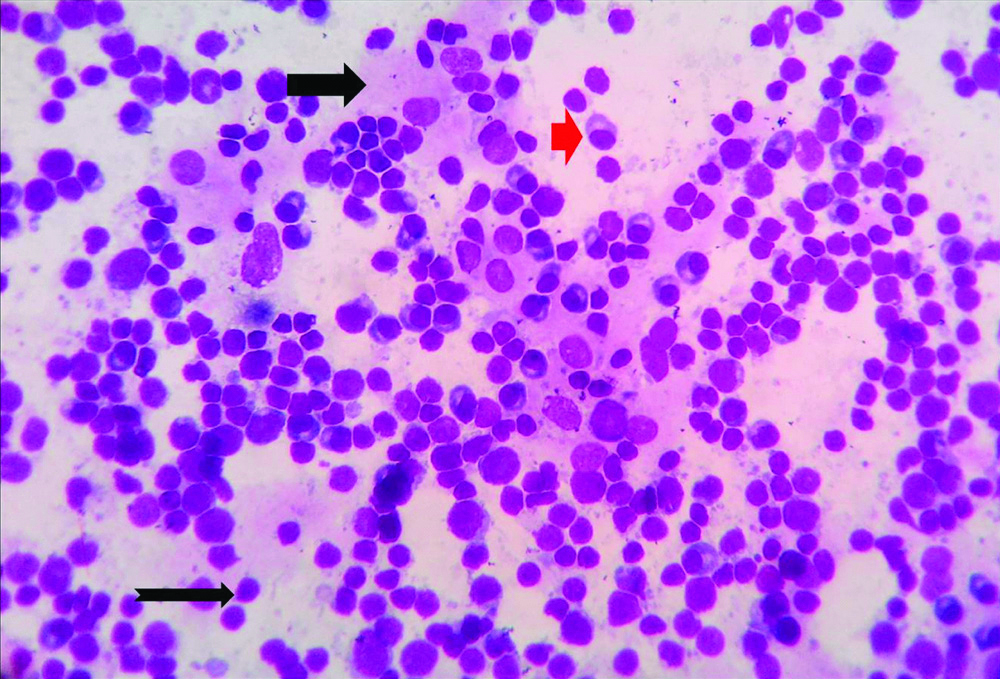
Microphotograph of peritoneal washing fluid cytology smear of gastric adenocarcinoma (Case 32) showing malignant cell displaying mitosis (Thick black arrow) and benign mesothelial cell (notched black arrow) against a background of numerous erythrocytes (Control slide, H&E, X400).
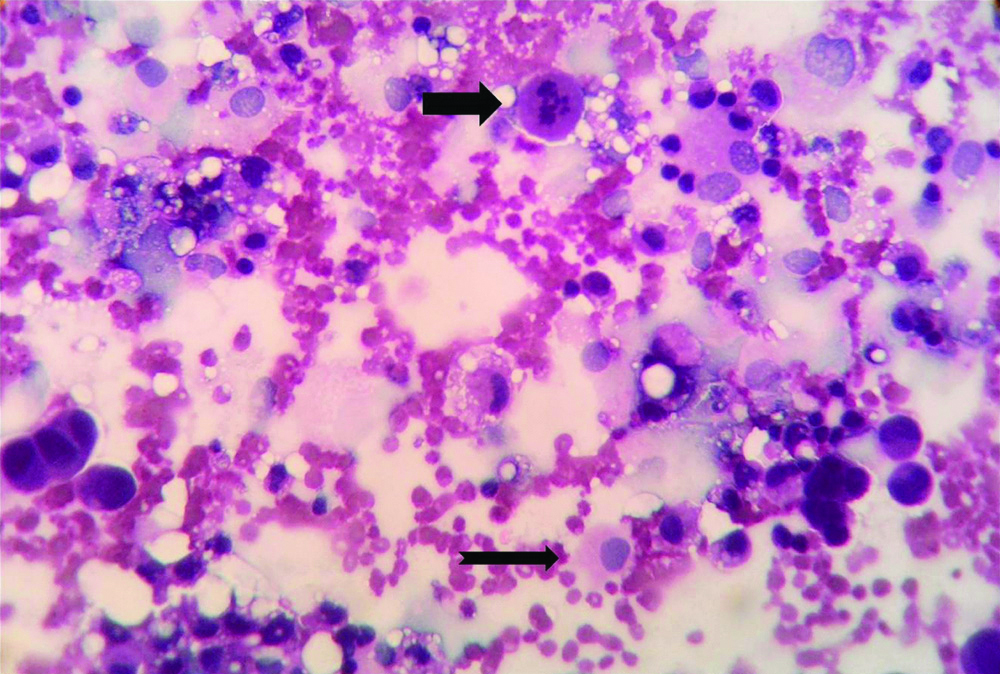
Microphotograph of peritoneal washing fluid cytology smear of a case of gastric adenocarcinoma (Case 32) showing malignant cell having enlarged nucleus (Thick black arrow) and mitosis (Notched black arrow) against a background of few erythrocytes (CF, H&E, X400).
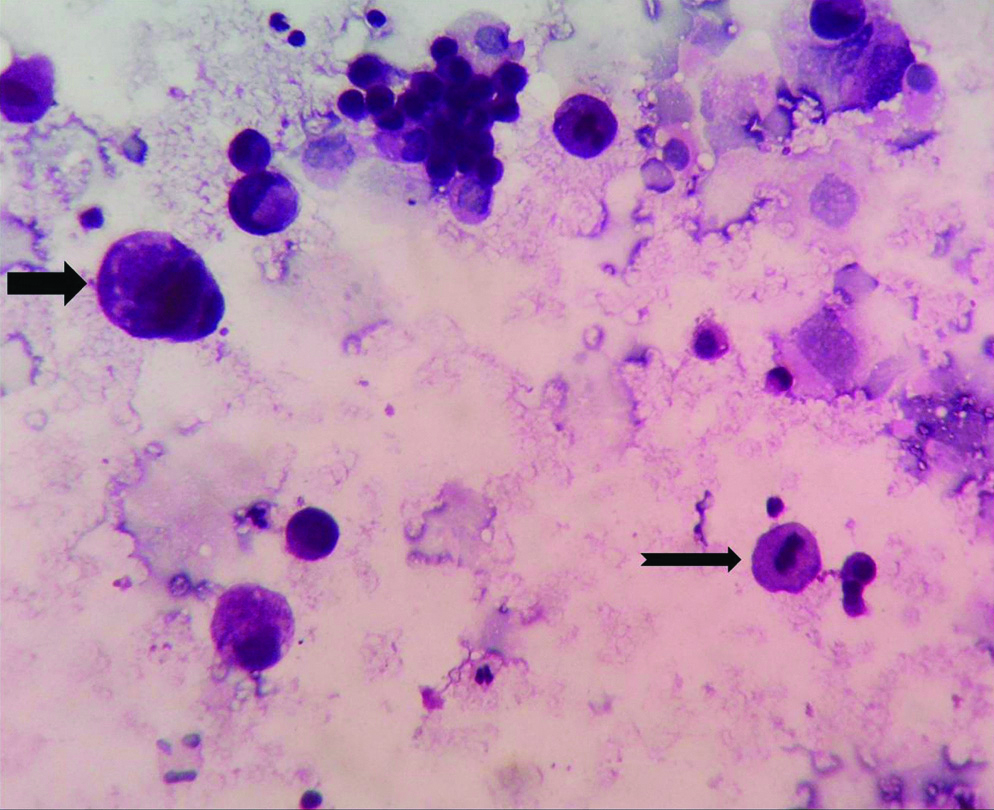
Microphotograph of peritoneal washing fluid cytology smear of gastric adenocarcinoma (Case 32) showing malignant cell having enlarged nucleus (Thick black arrow) and malignant cell displaying mitosis (Notched black arrow) against a relatively clear background (NSRT, H&E, X400).
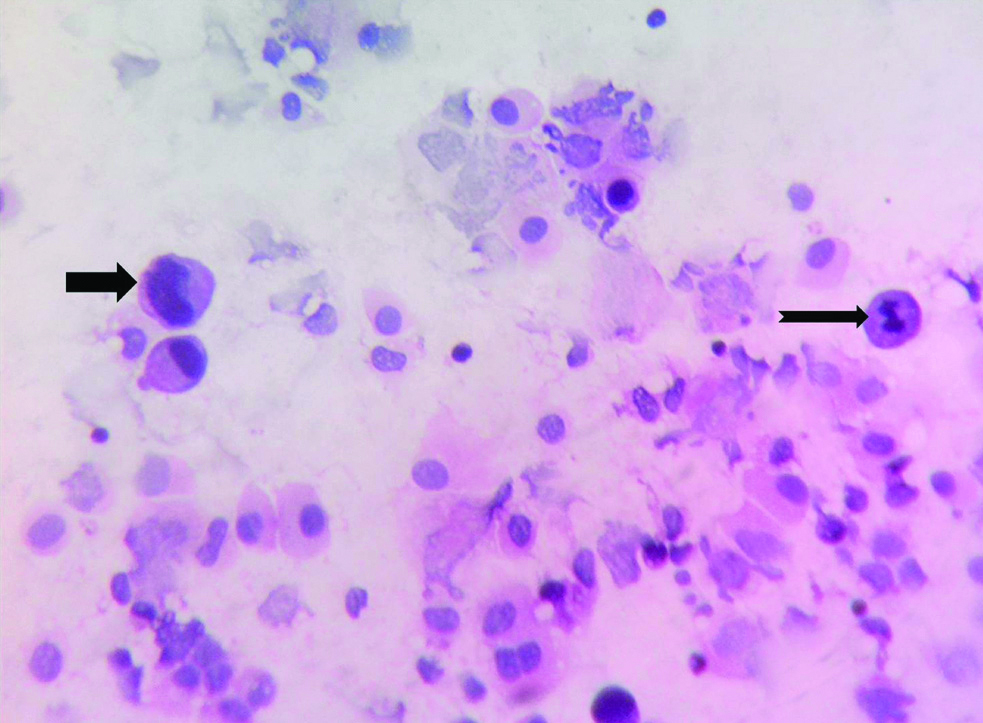
Microphotograph of peritoneal washing fluid cytology smear of gastric adenocarcinoma (Case 32) showing malignant cell displaying enlarged nucleus and cytoplasmic vacuolation (Thick black arrow) cluster against a relatively clear background (LSRT, H&E, X400).
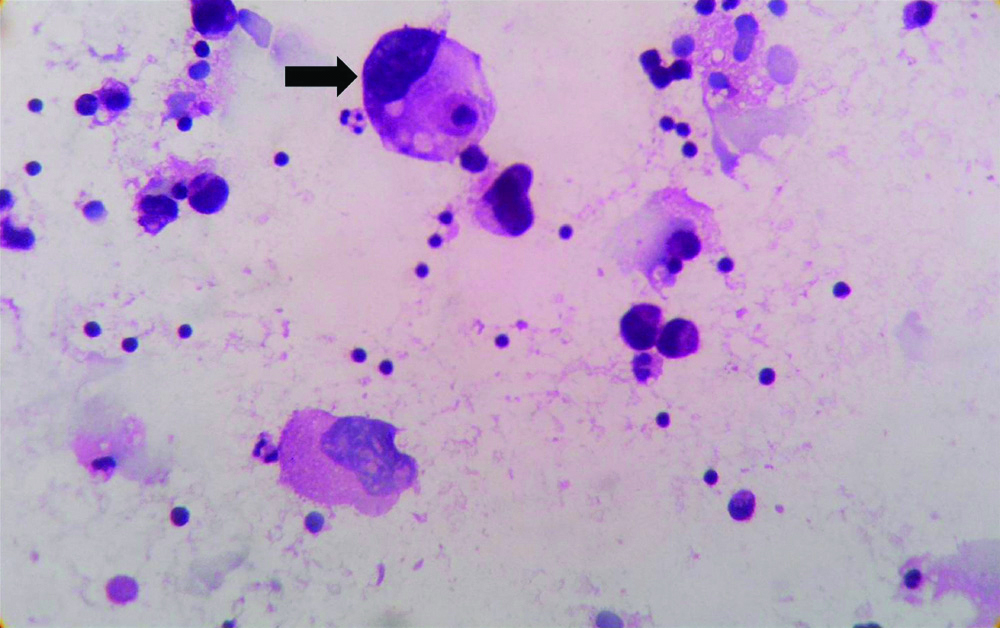
Overall Performance of Haemolysing Solutions
Best overall performance was observed with LSRT in both H&E smears and MGG smears, followed by NSRT. CF showed satisfactory overall performance. LSRT showed better average score than CF with respect to background (haemolysis) and retention of cells [Table/Fig-5].
Discussion
FNAC and body fluid cytology are simple, fast and accurate methods of obtaining material for morphological diagnosis. These techniques are especially effective for detecting malignancies; particularly metastatic cancers and staging of cancers. The diagnostic utility of these techniques suffer, when large number of RBCs are present in the specimen [7]. Several fixation methods have been devised for handling haemorrhagic samples, with an aim to fix the cells and create a background free of blood [8]. The present study involves development of LSRT, a new rehydration technique, to improve the quality of processing haemorrhagic samples. The new technique was compared with the known processing techniques (CF and NSRT).
In the present study, three haemolysing solutions (CF, NSRT and LSRT) were compared to analyse the effects of haemolysing agents. Shabnam M et al., Preeti et al., Malvi SG and Anthony IP, Rajput JS et al., used CF, GAA and NSRT to analyse the effects of haemolysing agent [1,2,8,9].
In the present study, Scoring was done on both MGG stained smears and H&E stained smears separately. The scores were recorded separately for each staining procedure. In the study conducted by Preethi et al., the scoring was done on PAP stained smears [2]. But, the other studies had not specified the staining procedure on which the scoring system was applied.
Effect of Haemolysing Solutions on Smear Background
In the present study, irrespective of the processing technique used, relatively lower percent of haemolysis was seen in few thyroid FNAC cases. It may be attributed to the presence of colloid material. The effect of CF on smear background was similar to the observations of Malvi SG et al., [8]. Best score (score 4) for haemolysing effect in CF showed less percentage of cases in both the studies. In contrast, other studies showed more percentage of cases for the best score. Best score for haemolysing effect in NSRT showed similar percentage of cases in studies conducted by Preethi et al., and Malvi SG and Anthony IP [2,8]. In contrast, the percentage of cases for the best score was in different range in the present study and other studies [Table/Fig-15]. The difference in observation can be attributed to the subjective nature of the scoring system.
Comparison of effect of haemolysing solutions on smear background, retention of cells and cytomorphological details in various studies.
| Comparison of effect of haemolysing solutions on smear background |
|---|
| Sl. No. | Authors | No. of cases | Stain used | Best scores [Score 4] |
|---|
| GAA | Carnoy’s fixative | NSRT | LSRT |
|---|
| 1 | Rajput JS et al., [9] | 76 | H&E, PAP and Leishman | 3 cases (4.16%) | 42 cases (58.39%) | 53 cases (73.81%) | - |
| 2 | Shabnam M et al., [1] | 51 | H&E, PAP and Leishman | 2 cases (3.9%) | 31 cases (60.8%) | 37 cases (72.5%) | - |
| 3 | Preeti et al., [2] | 150 | PAP | 80 cases (53.33%) | 123 cases (82%) | 137 cases (91.33%) | - |
| 4 | Malvi SG and Anthony IP [8] | 30 | PAP | 3 cases (10%) | 6 cases (20%) | 28 cases (93%) | - |
| 5 | Present study | 57 | MGG | - | 14 cases (24.56%) | 45 cases (78.95%) | 44 cases (77.19%) |
| H&E | - | 8 cases (14.04%) | 24 cases (42.11%) | 32 cases (56.14%) |
| Comparison of effect of haemolysing solutions on retention of cells |
| Sl. No. | Authors | No. of cases | Stain used | Best scores [Score 4] |
| GAA | Carnoy’s fixative | NSRT | LSRT |
| 1 | Rajput JS et al., [9] | 76 | H&E, PAP and Leishman | 42 cases (58.33%) | 48 cases (66.66%) | 38 cases (52.77%) | - |
| 2 | Shabnam M et al., [1] | 51 | H&E, PAP and Leishman | 26 cases (50.9%) | 29 cases (57.8%) | 36 cases (70.5%) | - |
| 3 | Preeti et al., [2] | 150 | PAP | 85 cases (56.65%) | 108 cases (72. 02%) | 130 cases (86.65%) | - |
| 4 | Present study | 57 | MGG | - | 42 cases (73.68%) | 35 cases (61.40%) | 49 cases (85.96%) |
| | | H&E | - | 43 cases (75.44%) | 36 cases (63.16%) | 50 cases (87.72%) |
| Comparison of effect of haemolysing solutions on cytomorphological details |
| Sl. No. | Authors | No. of cases | Stain used | Best scores (Score 4) |
| GAA | Carnoy’s fixative | NSRT | LSRT |
| 1 | Rajput JS et al., [9] | 76 | H&E, PAP and Leishman | 31 cases (43.05%) | 44 cases (61.11%) | 52 cases (72.22%) | |
| 2 | Shabnam M et al., [1] | 51 | H&E, PAP and Leishman | 30 cases (58.8%) | 31 cases (60.6%) | 27 cases (52.9%) | |
| 3 | Preeti et al., [2] | 150 | PAP | 102 cases (68%) | 116 cases (77.33%) | 70 cases (46.67%) | |
| 4 | Present study | 57 | MGG | - | 7 cases (12.28%) | 2 cases (3.51%) | 4 cases (7.02%) |
| H&E | - | 5 cases (8.77%) | 1 case (1.75%) | 2 cases (3.51%) |
Effect of Haemolysing Solutions on Retention of Cells
In the present study, the effect of CF on retention of cells was similar to the observations of Preeti et al., [2]. However, the percentage of cases for the best score was in different ranges in the other studies. The percentage of cases for the best score showed wide variation in different studies. [Table/Fig-15]. The difference in observation can be attributed to the subjective nature of the scoring system.
Most of the studies focused on epithelial cells and mesothelial cells for assessment of cellular retention. In contrast, the present study considered even the inflammatory cells, in addition to epithelial cells and mesothelial cells. In cytological evaluation of various entities, inflammatory cells would be helpful to decide whether a condition is acute condition (neutrophils) or a chronic condition (lymphocytes, macrophages). The presence of only inflammatory cells may also help us to rule out malignancy in the smears studied.
Effect of Haemolysing Solutions on Cytomorphological Details
In the present study, the percentage of cases for the best score in CF and NSRT was quiet low. In contrast, other studies observed higher percentage of cases for CF. The percentage of cases for the best score in NSRT in other studies was lower in comparison with CF, but varied widely in range. [Table/Fig-15]. The difference in observation can be attributed to the nature of the scoring system.
The reason for low percentage of cases for the best score in CF, NSRT and LSRT was attributed to difference in the effect of haemolysing agent on nucleus and cytoplasm of the cells. The nucleus may show very well preserved morphology and sharp features. On the other hand, the cytoplasm may not show well preserved morphology due to the effect of haemolysing solutions. Both the nature of nucleus and the nature of cytoplasm were taken into consideration to decide cytomorphological feature. Though the best score was low, average score was satisfactory for all the techniques.
For smear background, scores were better in MGG smears than H&E smears. But, for retention of cells, scores were better in H&E smears than in MGG smears. The probable reason for such difference in scores could be due to the extra time spent for drying the slides before staining the smear by MGG stain.
The lemon solution (10%) had specific gravity of 1.017 (determined by urinometer) and pH of 6 (determined by urine reagent dipstick). The solution may probably exert its effect by optimal osmotic effect to lyse the erythrocytes and preserve nucleated cells or by the virtue of its intrinsic physico-chemical properties.
In the present study, Chi-square test and Fischer-exact test were applied. The p-value was significant for all the effects of haemolysing solutions except for the cytomorphological details in H&E smears. Preeti et al., had also used chi-square test for interpreting all the values and p-value was significant for all the effects of haemolysing solutions [2]. On the contrary, Shabnam M et al., used ANOVA as statistical tool and values were not significant [1].
The modified scoring system, followed in many studies and the current study appears to be more subjective with respect to smear background and retention of cells. In the present study, haemolysis was not 100% by any of the three techniques used. Few non-haemolysed erythrocytes were seen even in the best scored smears. Furthermore, any of the three haemolytic solutions may have some lysing effect on erythrocytes. The effect may be negligible but not zero haemolysis. Hence, score 1 was not given in any of the techniques. Even in the studies conducted by Shabnam M et al., and Rajput JS et al., no cases were documented in score 1 [1,9]. The original scoring system given by Ng WF et al., gives only the specific percentage for different scores [4]. Modified scoring system mentions only approximate percent for haemolysis and cellular retention. But the scoring system does not specify exact range. The scores for cytomorphological details were well defined. But, it becomes difficult to decide the score if there is discrepancy between the appearance of nucleus and cytoplasm. This could be reason for wide variation in the percentage of best scores among different studies. Hence the scoring system needs to be further modified. A new scoring system may be proposed for the evaluation of smear treated with haemolysing solutions, so that the distribution of scores becomes more feasible and reproducible for future studies of such kind.
The rehydration technique has been extended to cervical smear in several studies [10-13]. Zare-Mirzaie A et al., found that air drying and rehydration of slides was a superior method for heavily blood stained smears and recommended its use for routine purpose [10]. Gupta S et al., and Sivaraman G et al., found that rehydration of air dried Pap smears was a suitable alternative to wet-fixed smears [12,13].
The limitation of NSRT is that if the smear is treated for more than five minutes causes blurring of cellular features. This can be avoided by carefully following the timing. NSRT affects the morphology of lymphoid cells as they do not have the same intermediate cytoskeleton which epithelial cells possess [14]. In the present study, cytomorphological alteration was seen more with neutrophils than in other cells. Limitation of CF is due to its composition namely ethanol, GAA and chloroform [15-18]. Chloroform is very hazardous and should be used in a ventilated hood by wearing masks. Exposure to chloroform has been associated with cancer and reproductive toxicity [19]. GAA has been reported to be associated with reversible airway obstruction following exposure to large amounts. It can be considered as a sensitiser of bronchial asthma [20]. Furthermore, CF produces a dirty and distracting background [8]. Limitation of LSRT was the occasional presence of microscopic fruit pulp artefacts in the background in few cases [Table/Fig-6]. It is possible to overcome the limitation by proper filtration of concentrated lemon juice using qualitative Whatman filter paper No.1. The only contraindication may be allergy to citrus fruit. Health care professionals allergic to citrus fruits may be advised to refrain themselves from performing the procedure. However, no such untoward incidence was encountered in the present study. The quality of lemon may vary in different places. Hence the concentration of the lemon solution needs to be standardised to obtain optimal results in a laboratory.
Limitation(s)
The number of cases analysed in the present study was relatively less in comparison with other studies. This was because the study was conducted only for a period of two months duration.
LSRT is a reliable, feasible and simple technique. It utilises lemon solution, a ubiquitous and non-hazardous natural product. It can be easily prepared in-house, even in low resource laboratories and ensures safety in laboratory practice. It can be utilised to overcome the disadvantages of Carnoy’s fixative and NSRT in the cytopathology laboratory.
Conclusion(s)
LSRT is a novel rehydration technique developed by using 10% lemon solution to improve the quality of processing haemorrhagic cytology samples. Cytomorphological details were better appreciated in LSRT than NSRT. LSRT showed best overall performance among the three processing techniques and ensures laboratory safety. It may be utilised to overcome the disadvantages of CF and NSRT. The current scoring system needs to be further modified. A new scoring system may be proposed, so that the distribution of scores becomes more feasible and reproducible for future studies of such kind.
Presentation
The research work was presented in the Dr. K.C.Basu Mallick Award Session at 67th Annual conference of Indian Association of Pathologists and Microbiologists (APCON 2018) held at Bareilly from 30th November to 2nd December 2018.