Gastroesophageal Reflux Disease (GERD) is one of the most common conditions worldwide with a significant impact on quality of life. The prevalence of GERD in India ranges from 5% to 18.7%, and it is associated with short-term as well as long-term consequences [1-4]. The most common symptoms of GERD are heartburn and regurgitation. Dyspepsia is also seen in many patients. Therapeutic management of these symptoms with proton pump inhibitors is challenging, as a significant number of patients remain unsatisfied. Therefore, clinicians include antacids or antiflatulent agents in the treatment regimen.
Almagate or simethicone alone have been used as add-on therapy to relieve the typical symptoms of GERD. Simethicone is used as an antiflatulent. It is an anti-foaming agent that decreases the surface tension of gas bubbles, resulting in the formation of larger bubbles in the digestive tract. It eases the release of gas within the gastrointestinal tract via burping or flatulence and does not reduce or prevent the formation of gas. On the contrary, almagate acts as an antacid that lowers the acid levels in the stomach. Literature suggests that almagate has a higher acid neutralising capacity with a buffering capacity in pH 3-5 making it effective for longer duration. It also has inhibition activity against pepsin with ability to adsorb bile acids and has low sodium content. It has good thermal stability as well as a rapid acid neutralisation velocity and a high acid consuming capacity. Therefore, almagate is an effective antacid drug as compared to other antacids [5,6].
Tricaine Alma is an oral suspension that contains a combination of almagate (500 mg) and simethicone (25 mg). The present post-marketing observational study (Treatment of Regurgitation in Indian patients: ALMA study {TRIALMA study}) was conducted to evaluate the safety and efficacy of almagate and simethicone combination in the symptomatic management of GERD.
Materials and Methods
A prospective, post-marketing observational, multicenter (n=42) study was conducted in patients with symptomatic GERD, between January 2019 and May 2019.
Patients aged between 18 and 65 years, with uncomplicated symptomatic GERD, frequent episodes of heartburn, regurgitation or dyspepsia symptoms, and those who required add-on antacid therapy were included in the study. Patients with complicated GERD, esophageal stricture, acute peptic ulcer, indication for Helicobacter pylori eradication therapy, any severe diseases of other body systems, or patients with any existing conditions that may compromise safety or participation in the study were excluded. Permission was obtained from each hospital authority. All the patients who reported to the respective hospitals during the study period were considered for the study. The study drug was prescribed to the patients as per the judgment of the prescribing physician.
Complete medical history was taken and physical examination was performed, and the patients were asked if they had been receiving any concomitant medication. All of the information provided by patients was noted.
All patients received suspension of almagate and simethicone combination during their initial visit (5-10 mL thrice a day for 15 days). The intensity of heartburn, regurgitation, and dyspepsia was recorded using Visual Analog Scale (VAS) of 0 (no symptoms) to 10 (severe symptoms) at baseline and after 15 days (during the follow-up visit or telephonic assessment) for symptomatic relief.
Overall patient satisfaction score (ranging from 1=don’t like at all, 2=don’t like it, 3=don’t know, 4=like it, 5=like it very much) was assessed and adverse events were recorded, if any, at the end of the study after 15 days of treatment. All the patients who agreed to participate in the study were requested to follow-up after 15 days. Patients who visited 2 days before or after the scheduled visit were considered compliant and included in the final analysis. All other patients were excluded.
Statistical Analysis
All data were analysed using Statistical Package for Social Sciences (SPSS) software version 23.0. All categorical and ordinal variables were expressed as frequencies and percentages. The continuous variables were expressed as mean {Standard Deviation (SD)} and compared by Mann-Whitney U test. A p-value <0.05 was considered as significant.
Results
A total of 1812 patients were enrolled in the study, of which 45 patients discontinued the treatment. Data of remaining 1767 patients who continued the treatment were analysed.
The mean (SD) age of patients was 43.59 (14.60) years. A total of 793 (44.9%) patients were male and 974 (55.1%) were female. The mean (SD) overall satisfaction score was 4.31 (0.66). Thirty-eight patients (2.15%) reported adverse events after treatment [Table/Fig-1].
Characteristics of study population.
| Patient characteristics | Overall patients (n=1812) | Patients who continued the treatment (n=1767) |
|---|
| Age (years) | 43.67 (14.60) | 43.59 (14.60) |
| Gender, n (%) |
| Male | 816 (45) | 793 (44.9) |
| Female | 996 (55) | 974 (55.1) |
| Overall satisfaction score | 4.14 (0.93) | 4.31 (0.66) |
| Adverse drug reaction reported, n (%) | 40 (2.20) | 38 (2.15) |
Data shown as mean (SD), unless otherwise specified
Scoring of symptoms at baseline and after 15 days is shown in [Table/Fig-2]. At baseline, regurgitation (n=1762) was the most common reflux-related symptom followed by heartburn (n=1757) and dyspepsia (n=1754). The mean (SD) symptom score for heartburn at baseline {9.09 (1.24)} was significantly reduced after 15 days of treatment {1.01 (1.49); p=0.047}. The mean (SD) symptom score for regurgitation was significantly decreased after 15 days of treatment compared with baseline {8.98 (1.19) vs. 0.99 (1.21); p<0.001}. The mean (SD) symptom score for dyspepsia was significantly reduced after 15 days of treatment {0.83 (0.93)} compared with baseline {8.92 (1.30); p=0.003}.
Scoring of symptoms at baseline and after 15 days in patients who continued the treatment (n=1767).
| Symptoms | Baseline score | After 15 days score | p-value |
|---|
| Heartburn | 9.09 (1.24) | 1.01 (1.49) | 0.047 |
| Regurgitation | 8.98 (1.19) | 0.99 (1.21) | <0.001 |
| Dyspepsia | 8.92 (1.30) | 0.83 (0.93) | 0.003 |
Data shown as mean (SD)
Statistical test used: Mann-Whitney U test
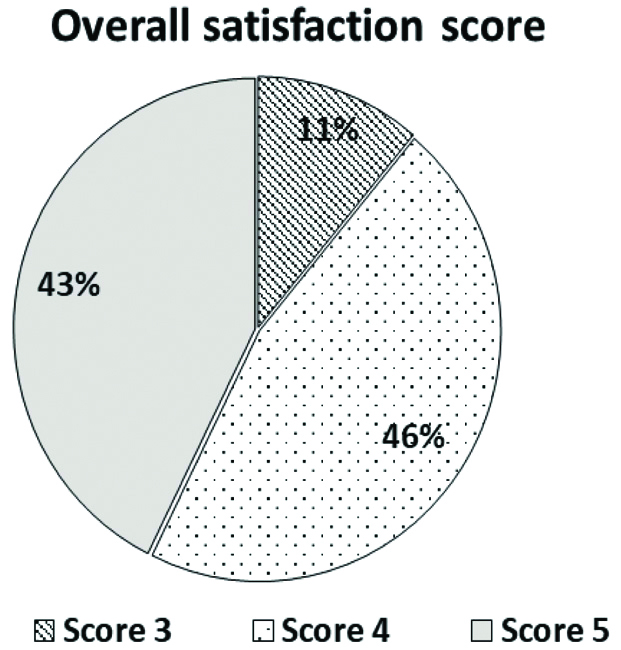
Post-treatment, most of the patients (n=817; 46%) had an overall satisfaction score of 4 followed by a satisfaction score of 5 (n=754; 43%) and 3 (n=196; 11%) [Table/Fig-3].
Overall satisfaction score after 15 days of treatment.
[Table/Fig-4] shows a comparison of symptoms score at baseline vs. after 15 days of treatment according to the overall satisfaction score. Patients with an overall satisfaction score of 3 showed significantly reduced mean symptoms score after 15 days of treatment compared with baseline for heartburn (8.88 vs. 2.11), regurgitation (8.69 vs. 1.84), and dyspepsia (8.44 vs. 1.58) after 15 days of treatment (p<0.001). The mean symptoms score for heartburn, regurgitation and dyspepsia was significantly reduced from baseline (8.88, 8.91 and 8.88, respectively) to post 15 days of treatment (1.29, 1.23 and 0.97, respectively) in patients with an overall satisfaction score of 4 (p<0.001). Patients with overall satisfaction score of 5 had a significantly lower mean symptom score after 15 days of treatment for heartburn (9.37 vs. 0.41), regurgitation (9.11 vs. 0.61), and dyspepsia (9.08 vs. 0.48) from baseline (p<0.001).
Comparison of symptoms score at baseline versus after 15 days according to overall satisfaction score in patients with GERD (n=1767).
| Overall satisfaction score | Baseline score | After 15 days score | p-value |
|---|
| Heartburn | Regurgitation | Dyspepsia | Heartburn | Regurgitation | Dyspepsia |
|---|
| 1 | - | - | - | - | - | - | - |
| 2 | - | - | - | - | - | - | - |
| 3 | 8.88 (2.17) | 8.69 (2.00) | 8.44 (2.00) | 2.11 (2.73) | 1.84 (2.05) | 1.58 (1.36) | <0.001a,b,c |
| 4 | 8.88 (1.15) | 8.91 (0.98) | 8.88 (1.16) | 1.29 (1.24) | 1.13 (1.02) | 0.97 (0.79) | <0.001a,b,c |
| 5 | 9.37 (0.92) | 9.11 (1.09) | 9.08 (1.19) | 0.41 (0.91) | 0.61 (0.92) | 0.48 (0.76) | <0.001a,b,c |
Data shown as mean (SD); aheartburn (baseline vs. after 15 days); bregurgitation (baseline vs. after 15 days); cdyspepsia (baseline vs. after 15 days); Statistical test used: Mann-Whitney U test
A small proportion of patients (n=38, 2.15%) reported mild adverse events after treatment. The common adverse events were headache, stomach upset, and diarrhea, which were resolved in 2-3 days with supplementary medications.
Discussion
The present study evaluated effectiveness and tolerability of almagate and simethicone combination in patients with uncomplicated symptomatic GERD. The patients included in this study had been experiencing frequent episodes of heartburn, regurgitation, or dyspepsia symptoms and required add-on treatment with antacids to alleviate their pain and uneasiness. Therefore, they were administered an add-on treatment with a combination of antacid (almagate 500 mg) and antiflatulent (simethicone 25 mg) for 15 days.
The mean age of the patients was 43.59 years and 55.1% were females. The average overall satisfaction score reported was 4.31, which suggests that the patients liked the treatment and were satisfied with the symptomatic relief that they experienced.
In this study, regurgitation was the highest reflux-related symptom observed followed by heartburn and dyspepsia. The severity of symptoms was measured by visual analog scale at baseline and after 15 days of treatment to analyse the effectiveness of this treatment. These results indicate that all the symptoms were severe before initiation of the treatment. After 15 days of treatment, all the symptoms scores were significantly reduced (to almost no symptoms levels. Therefore, the combination of almagate and simethicone was efficacious in relieving the patients from severe symptoms of heartburn, regurgitation, and dyspepsia.
The present study also compared the symptoms score at baseline to that after 15 days of treatment according to the overall satisfaction score. The mean symptoms score for heartburn, regurgitation, and dyspepsia was significantly reduced from baseline to after 15 days of treatment in patients with overall satisfaction score of 3, 4. and 5. In patients with an overall satisfaction score of 5, the reduction in symptoms score was maximum suggesting the negative correlation between symptoms score and overall satisfaction of patients.
A recent study by Wilkinson J, et al., assessed the efficacy and safety of Gaviscon DA, which has a combined acid-neutralising and reflux suppressing action resulting in the reduction of upper gastrointestinal symptoms in patients with GERD [7]. They reported that Gaviscon DA, is effective and well-tolerated among patients with reflux symptoms and associated dyspepsia in symptomatic GERD patients.
There are no similar studies available to compare the results observed in the present study. However, there is one study that evaluated the effect of almagate on gastroesophageal reflux in oesophagitis patients, and it suggested that the almagate could be effective in the treatment of GERD, since the acidity profile of patients treated with it has been shown as an intragastric long-lasting alkalinisation wave [8]. Previous studies have shown efficacy and safety of simethicone in the treatment of functional bloating [9,10].
Other antacids have also been studied in various combinations in GERD patients. A recent phase 4 randomised controlled study compared the efficacy and safety of omeprazole-domperidone combination vs. omeprazole monotherapy in GERD. They showed better efficacy and safety of omeprazole-domperidone combination over omeprazole alone in providing complete cupping of reflux symptoms and healing of oesophagitis and significantly longer period of heartburn-free days [11]. Another multicentric post marketing observational study demonstrated that a significant decrease in severity of both typical and atypical GERD symptoms within the first 7 days of treatment (pantoprazole magnesium 40 mg) once daily which continued over 4 weeks [12]. This result indicates that the add-on treatment with almagate and simethicone was well tolerated by these patients.
Limitation(s)
There are a few limitations of this study. Firstly, this study did not record details of adverse events that occurred in patients. Secondly, the follow-up duration was very short which limited the authors to understand the long-term effect of the add-on treatment. Lastly, the premedications administered for management of GERD were not recorded or taken into consideration while analysing the data.
Conclusion(s)
These observations indicate that the add-on treatment with oral suspension of almagate and simethicone was effective in relieving of reflux-related symptoms and was well tolerated in patients with GERD.
Funding: The study was funded by RPG Life Sciences Ltd, Mumbai.
Declaration: Ethics Committee approval was not obtained for this study as this was a post-marketing observational study where data was collected from practitioners after seeking due permission.
Data shown as mean (SD), unless otherwise specified
Data shown as mean (SD)
Statistical test used: Mann-Whitney U test
Data shown as mean (SD); aheartburn (baseline vs. after 15 days); bregurgitation (baseline vs. after 15 days); cdyspepsia (baseline vs. after 15 days); Statistical test used: Mann-Whitney U test