Antineoplastic drugs when administered as recommended results in improved quality of life, overall survival and disease free survival. CIT is a common adverse effect resulting in treatment delays, increased health care cost and poorer treatment outcomes [1]. CIT is defined as platelet count <1,00,000/μL and has a prevalence of 1-25% in solid malignancies [2]. CIT is usually observed 6-14 days after a chemotherapy cycle [3]. The available treatment options for management of CIT include platelet transfusion, recombinant thrombopoietin [4] thrombopoietin receptor agonists Eltrombopag Romiplostim and recombinant Interleukin 11 (Oprelvekin) [5]. Repeated platelet transfusions is costly, causes transfusion reactions, alloimmunisation and infections [6]. Eltrombopag and romiplastim causes hepatotoxicity and are costly [7]. Oprelvekin has serious systemic toxicity. Hence there is an unmet need in CIT management to prevent delays and dose compromise of chemotherapy.
Materials and Methods
This was a single-institution; retrospective chart audit conducted at Amrita Institute of Medical Sciences, Kochi, Kerala over a period of six months (July-December 2018) among patients who received CPLE for CIT. It was a chart audit of institutional practice, for which an institutional permission was sort. There was no control arm for comparison. CPLE is commonly used in the institution for management of CIT. As per institutional protocol, all patients with proven CIT were administered CPLE 1100 mg tablet thrice daily until platelet recovery or up to a maximum of two weeks irrespective of the outcome [11]. This dose of CPLE is accepted standard as described in previous reported studies on dengue-induced thrombocytopenic patients. Platelet counts were generally checked on alternate days from Day 3 of CPLE administration up to platelet recovery.
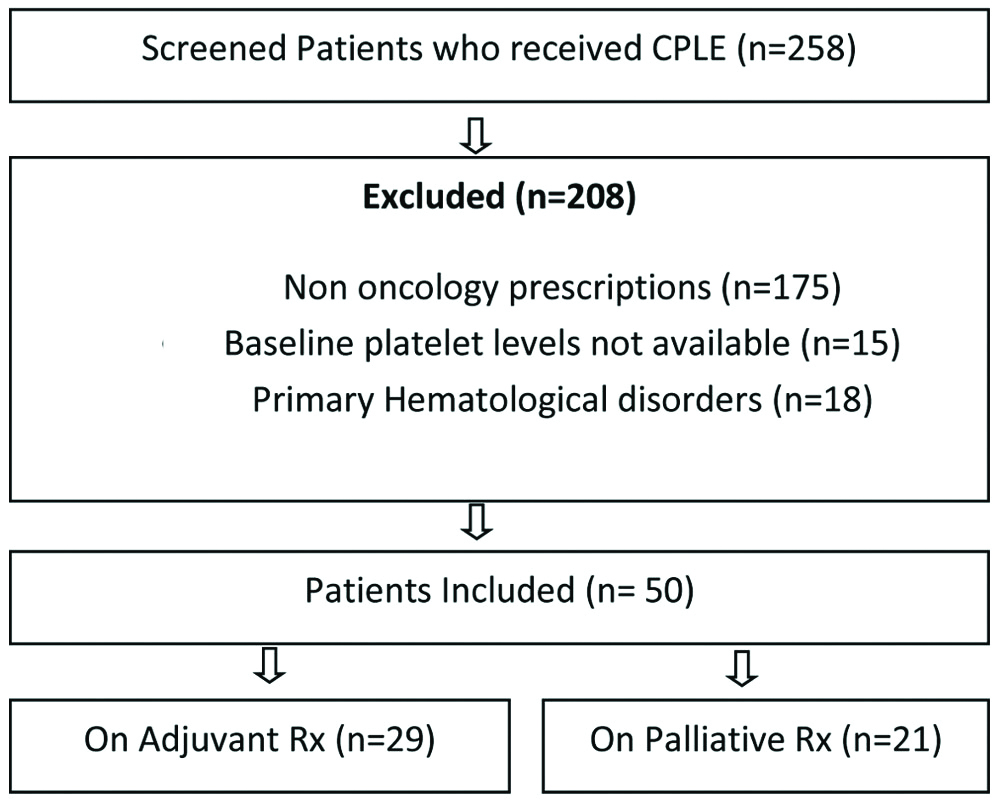
There were 258 patients who had been prescribed CPLE. All eligible patients were above the age of 18 years, with baseline normal thrombocyte count, undergoing adjuvant or palliative chemotherapy for solid tumours, and presenting with a platelet counts less than one hundred thousand per cubic millilitre, secondary to chemotherapy at the time of subsequent cycle. Patients with primary thrombocytopenia, known case of haematological malignancy and idiopathic thrombocytopenia purpura were excluded. Patients who satisfied the above criteria were included in this study [Table/Fig-1]. Since this was a retrospective study, patient consent was not required.
Schematic representation of patient selection process.
Statistical Analysis
Numerical variables such as age, baseline platelet count, and platelet count before and after treatment, percentage volume increase were expressed as mean and standard deviation. Categorical variables like sex and type of chemotherapy provided were expressed as frequency and percentages. To test the statistical significance in mean values from baseline to follow-up period, Wilcoxon signed rank test was applied. To obtain the correlation between continuous variables Karl Pearson’s correlation coefficient was applied. Statistical analysis was carried out using the IBM SPSS Statistics for Windows, Version 20.0. (Armonk, NY: IBM Corp.).
Results
The study included a group of 50 patients of CIT [Table/Fig-1]. Patient details are tabulated in [Table/Fig-2]. Five patients had two episodes of CIT during the course of chemotherapy. Twenty-four patients were males. The median age of patients was 56.5 years (range 19-75 years). Fifteen patients were above the age of sixty years. The disease spectrum included fourteen Central Nervous System (CNS) tumours (eleven glioblastoma multiforme, three anaplastic oligodendroglioma and one primary CNS lymphoma). Seven patients had gynaecologic malignancy. Six patients had colorectal disease; four each had breast and upper digestive tract malignant diseases. Three patients had head and neck tumours, two each had lymphoma, lung and urothelial malignancy and one patient had thymic carcinoma. Twenty nine patients (58%) were receiving adjuvant chemotherapy. Remaining twenty one patients (42%) were receiving treatment for recurrent and metastatic disease. All the patients had a normal baseline blood count including platelet count. The mean baseline platelets count was 2.98 Lacs. The chemotherapy drugs used were Temozolomide, Paclitaxel, Docetaxel, Gemcitabine, Doxorubicin, Cyclophosphamide, Rituximab, Vincristine, 5-fluorouracil, Cisplatin, Carboplatin, Oxaliplatin and Capecitabine.
| Sl No. | Age/Sex | Diagnosis | Adjuvant/Palliative (With line of treatment) | Lowest platelet count noted in cycle | Pre chemo baseline platelet count (per microliter) | Platelet count before CPLE treatment (per microliter) | Total days taken for recovery | Platelet count after treatment (per microliter) | % Volume increase in platelet |
|---|
| 1 | 42/M | NASOPHARYNX | ADJ | 3 | 159000 | 90000 | 7 | 108000 | 20 |
| 2 | 44/M | CA TONGUE | ADJ | 2 | 226000 | 95000 | 5 | 168000 | 77 |
| 3 | 40/M | CA TONGUE | ADJ | 1 | 102000 | 71000 | 17 | 118000 | 66 |
| 4 | 60/M | CA ESOPHAGUS | ADJ | 2 | 201000 | 31000 | 5 | 152000 | 390 |
| 5 | 66/M | CA STOMACH | ADJ | 3 | 282000 | 99000 | 5 | 93000 | -6 |
| 6 | 67/M | CA GE JUNCTION | ADJ | 4 | 275000 | 77000 | 5 | 116000 | 50 |
| 7 | 72/F | CA CAECUM | ADJ | 4 | 294000 | 32000 | 5 | 110000 | 243 |
| 8 | 54/F | CA COLON | ADJ | 3 | 224000 | 75000 | 13 | 160000 | 113 |
| 9 | 55/F | CA RECTUM | ADJ | 6 | 262000 | 90000 | 5 | 144000 | 60 |
| 10 | 62/M | CA PANCREAS | ADJ | 4 | 533000 | 75000 | 5 | 197000 | 162 |
| 11 | 46/M | CA THYMUS | ADJ | 2 | 326000 | 36000 | 13 | 192000 | 433 |
| 12 | 46/M | CA THYMUS | ADJ | 3 | 326000 | 87000 | 18 | 168000 | 93 |
| 13 | 61/M | GBM | ADJ | 2 | 263000 | 54300 | 19 | 131000 | 141 |
| 14 | 44/F | GBM | ADJ | 1 | 215000 | 15000 | 7 | 27000 | 80 |
| 15 | 66/M | ODG | ADJ | 2 | 386000 | 60000 | 5 | 116000 | 93 |
| 16 | 66/M | ODG | ADJ | 3 | 386000 | 38900 | 9 | 122000 | 213 |
| 17 | 51/F | GBM | ADJ | 5 | 259000 | 71000 | 5 | 144000 | 102 |
| 18 | 30/M | GBM | ADJ | 2 | 344000 | 44000 | 5 | 244000 | 454 |
| 19 | 19/M | GBM | ADJ | 3 | 235000 | 20000 | 5 | 3000 | -85 |
| 20 | 38/F | GBM | ADJ | 5 | 212000 | 87000 | 5 | 145000 | 66 |
| 21 | 38/F | GBM | ADJ | 6 | 212000 | 92000 | 5 | 126000 | 37 |
| 22 | 58/F | GBM | ADJ | 5 | 271000 | 37000 | 5 | 11000 | -70 |
| 23 | 51/F | GBM | ADJ | 4 | 123000 | 91000 | 5 | 170000 | 86 |
| 24 | 42/M | PCNSL | ADJ | 6 | 196000 | 67000 | 13 | 161000 | 140 |
| 25 | 43/F | CA BREAST | ADJ | 4 | 278000 | 51000 | 5 | 232000 | 354 |
| 26 | 57/F | CA BREAST | ADJ | 1 | 332000 | 79000 | 3 | 215000 | 172 |
| 27 | 53/F | CA OVARY | ADJ | 2 | 654000 | 59000 | 5 | 260000 | 340 |
| 28 | 65/F | CA OVARY | ADJ | 1 | 253000 | 19000 | 7 | 107000 | 463 |
| 29 | 58/F | CA CERVIX | ADJ | 5 | 396000 | 82000 | 7 | 156000 | 90 |
| 30 | 66/F | ODG | PAL 1 | 2 | 477000 | 15200 | 9 | 172000 | 1031 |
| 31 | 37/F | GBM | PAL 1 | 4 | 185000 | 58000 | 5 | 31000 | -46 |
| 32 | 57/M | CA LUNG | PAL 1 | 6 | 353000 | 20000 | 7 | 110000 | 450 |
| 33 | 35/F | CA BREAST | PAL 1 | 5 | 259000 | 95000 | 7 | 260000 | 173 |
| 34 | 60/F | CA OVARY | PAL 1 | 4 | 705000 | 41000 | 7 | 133000 | 224 |
| 35 | 52/F | CA OVARY | PAL 1 | 1 | 587000 | 61000 | 7 | 211000 | 245 |
| 36 | 56/M | CA COLON | PAL 1 | 6 | 340000 | 75000 | 9 | 73000 | -2.6 |
| 37 | 57/M | CA RECTUM | PAL 1 | 5 | 280000 | 75000 | 13 | 100000 | 33 |
| 38 | 65/M | CA UROTHELIAL | PAL 1 | 4 | 189000 | 55000 | 5 | 150000 | 190 |
| 39 | 75/M | CA UROTHELIAL | PAL 1 | 6 | 341000 | 25000 | 11 | 140000 | 460 |
| 40 | 58/M | GBM | PAL 2 | 2 | 187000 | 87000 | 7 | 81000 | -6.8 |
| 41 | 60/F | CA BREAST | PAL 2 | 2 | 411000 | 85000 | 5 | 303000 | 256 |
| 42 | 55/F | CA LUNG | PAL 2 | 1 | 278000 | 53000 | 5 | 150000 | 183 |
| 43 | 55/F | CA LUNG | PAL 3 | 1 | 278000 | 32000 | 5 | 325000 | 915 |
| 44 | 60/F | CA BREAST | PAL 2 | 4 | 287000 | 75000 | 6 | 237000 | 216 |
| 45 | 70/F | OVARY | PAL 2 | 3 | 339000 | 38000 | 13 | 135000 | 255 |
| 46 | 54/M | CA RECTUM | PAL 2 | 2 | 268000 | 75000 | 5 | 167000 | 122 |
| 47 | 54/M | CA RECTUM | PAL 2 | 3 | 268000 | 95000 | 5 | 178000 | 87 |
| 48 | 64/M | NHL | PAL 2 | 3 | 147000 | 68000 | 5 | 200000 | 194 |
| 49 | 64/M | NHL | PAL 2 | 5 | 147000 | 62000 | 5 | 131000 | 111 |
| 50 | 73/F | CA OVARY | PAL 4 | 1 | 379000 | 48000 | 13 | 83000 | 72 |
ADJ: Adjuvant; CA: Carcinoma; F: Female; GBM: Glioblastoma multiforme; M: Male; NHL: Non-hodgkins lymphoma; ODG: Anaplastic oligodendroglioma; PAL: Palliative (1,2,3,4 stands for the line of treatment); PCNSL: Primary CNS lymphoma
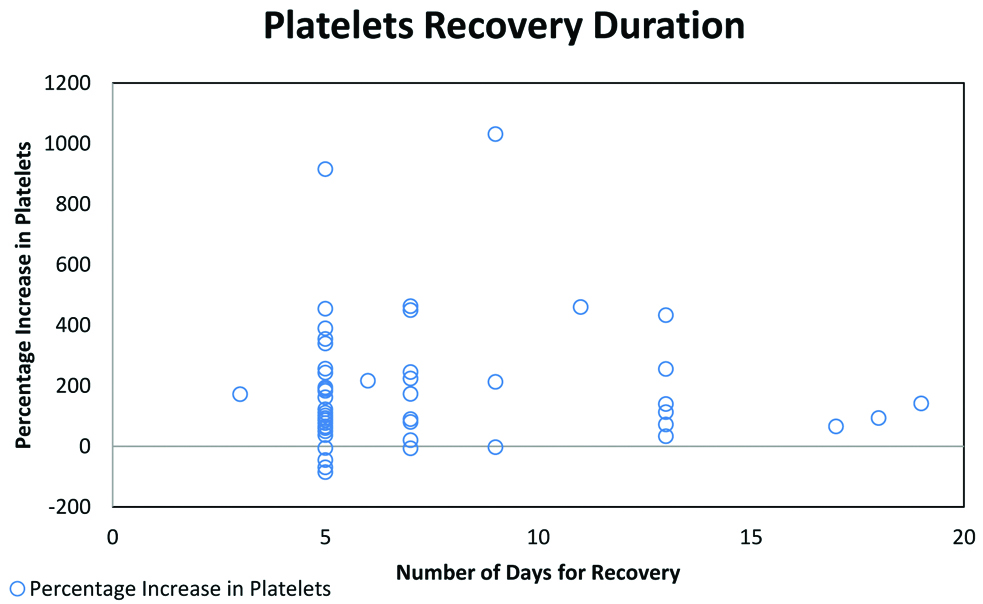
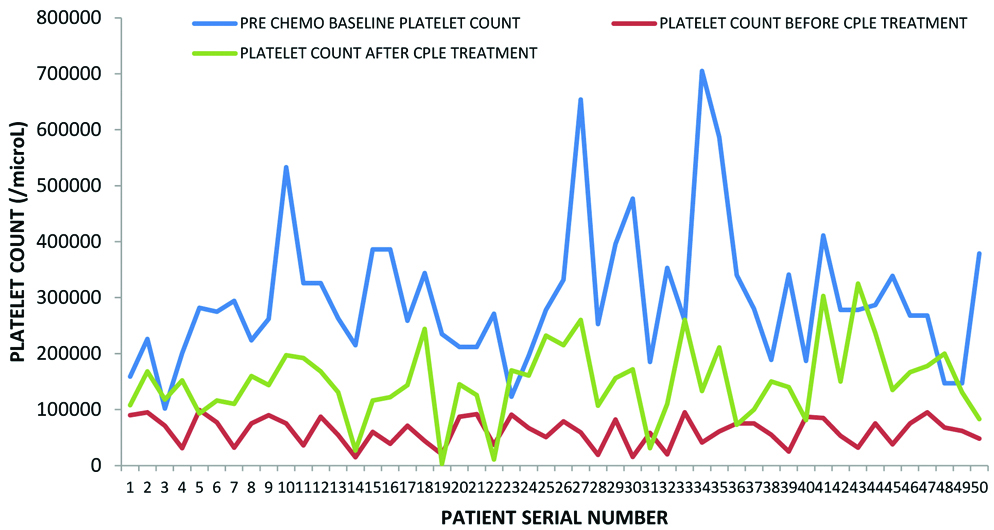
There was a negative correlation between age and the risk for thrombocytopenia suggesting that the risk of CIT increased with increasing age. A slightly higher number of patients on adjuvant therapy had CIT when compared to the patients on palliative therapy. This is likely due to reduced chemotherapy dosage and intensity of treatment in the patients on palliative intent compared to those who were on curative intent adjuvant therapy. Seventy four percent patients developed CIT in first 4 cycles of chemotherapy. The nadir mean platelet counts post-chemotherapy was 61268/cumm. Post-CPLE treatment the mean platelet counts was 149320/cu mm. There was a significant improvement in the platelet count in 54% (n=27) patients within 5 days and another 16% (n=8) by 7 days of CPLE administration [Table/Fig-3]. Eighteen percent (n=9) patients took longer than one week to recover their platelet counts. Twelve percent (n=6) patients had deteriorating platelet counts even after CPLE intervention, one among them mandating platelet transfusion. Four of those who had worsening platelets had Glioblastoma Multiforme (GBM) on temozolomide, one each were on treatment for colon and stomach cancer. Two patients improved, however they did not achieve normal platelet counts even after 2 weeks of CPLE administration. The overall improvement in the platelet counts on intervention with CPLE was significant (p<0.01) [Table/Fig-4]. Though not significant, there was a positive correlation between pre and post-treatment platelet values after CPLE (R score 0.2189; p=0.12). The only adverse effects that were encountered were dysgeusia and nausea which were of mild intensity (grade 1).
Represent total days taken for recovery from CIT. Majority of the patients recovered from CIT in between 5-7 days.
Represents platelet counts prior to chemotherapy (baseline), before (nadir) and after treatment with CPLE.
Discussion
Purified papain is known to induce IL-6 secretion in dose dependent manner in modified mixed human lymphocyte culture which directly stimulates thrombocyte production by increasing Thrombopoietin (TPO) secretion in the liver [9].
In two different studies in murine models Ahmed N et al., and Dharmaratna SLCA et al., have reported potential activity of CPLE in increasing thrombocyte count without causing significant systemic toxicity [12,13]. Even though, considered a traditional medicine, Subenthiran S et al in his study on DHF has shown that CPLE improves thrombocyte counts [14]. Sarala N and Paknikar S, reviewed seven published studies and concluded that CPLE is a good therapeutic option for thrombocytopenia in dengue haemorrhagic fever [15].
Bleeding secondary to CIT is an extremely rare phenomenon unlike DHF where thrombocytopenia combined with endothelial injury, activation of coagulation and fibrinolysis leads to dramatic and occasionally life threatening bleeding manifestations. The clinical efficacy and safety of CPLE for human use in DHF has been well documented through multiple published studies in clinical literature. Hence CPLE usage in medical oncology practice is probably only as an agent to attempt reducing treatment disruption secondary to CIT. There has been no major randomised control trial on CPLE in CIT. This was restrospective study with sole intent to determine the effect of CPLE on CIT. No other statistically relevant conclusions can be drawn from this study due to small sample size. A recent pilot study by Sundarmurthy D et al., from Kidwai Institute of Oncology has looked at the efficacy and safety of CPLE in oncology patients [10]. To the best of knowledge, the study by Kidwai is the only study to have analysed the use of CPLE in oncology practice. This audit attempts to add to this knowledge base.
The Kidwai Memorial study had randomised patients who had low platelet counts on Day 7-10 post chemotherapy to CPLE intervention and non-intervention group. The current study was retrospective in nature and therefore included only those patients who had not recovered their platelet counts on the due date of their next cycle of chemotherapy, which was commonly found to be 3-4 weeks from the previous cycle. The platelet nadir is generally seen between day 6-14 after chemotherapy, following which the platelet counts recover naturally. If the platelet count has not recovered by the day of next planned chemotherapy then it can be truly called delayed recovery of CIT thereby allowing us to evaluate the efficacy of CPLE. None of our patients were given primary prophylaxis with CPLE. Unlike Kidwai study, where all the patients responded, six (12%) of the present study patient’s platelet counts worsened. Three among them were being treated for recurrent disease and three were on adjuvant therapy. All these patients who had worsening platelet in spite of CPLE administration, were less than 60 years of age. The one with maximum drop in platelet count was 19 year-old who could not continue further chemotherapy. Two other patients did not achieve normal platelet counts even after 2 weeks of CPLE administration. This suggests that myelosuppression alone may not be the sole cause of thrombocytopenia. It can occur secondary to immune mediated mechanisms of chemotherapy agents, splenic sequestration due to liver toxicity, direct effect on platelet release, inhibition of PDGF and apoptosis of megakaryocytes, or release of megakaryocyte toxic mediators into the bone marrow milieu [16]. In patients with recurrent disease, there is a higher likelihood of non-response to CPLE which could be likely due to poor bone marrow reserves. Kidwai study reported increased platelet count by day 13. Compared to the Kidwai study, 54% of patients in this study recovered the platelet counts by the fifth day of administering CPLE. Further 16% patients recovered the platelet counts by the tenth day. This is representative of the effect of CPLE, as a rapid acceleration was seen in platelet counts after starting CPLE. There were no major adverse effects with the use of CPLE for up to two weeks.
Limitation(s)
This study was retrospective in nature and has small number of patients. It was only intended to be exploratory in nature to identify whether a treatment effect can be achieved with CPLE in CIT. There was no randomisation. The acceleration in platelet recovery with the used of CPLE is insufficient to recommend its routine use.
Conclusion(s)
CPLE has a modulatory effect on platelet production and therefore may be considered as a cost-effective option for management of CIT, particularly in situations where more established drugs like eltrombopag may not be available or affordable. A large well powered randomised study alone could answer this question.
ADJ: Adjuvant; CA: Carcinoma; F: Female; GBM: Glioblastoma multiforme; M: Male; NHL: Non-hodgkins lymphoma; ODG: Anaplastic oligodendroglioma; PAL: Palliative (1,2,3,4 stands for the line of treatment); PCNSL: Primary CNS lymphoma