Cancer is a major cause of death, for which early diagnosis and treatment remain the best strategy to improve prognosis and quality of life [1,2]. Improving understanding of the molecular mechanisms that underlie the malignant transformation of cancer cells may lead to the development of new therapeutic strategies to fight cancer [3]. Worldwide, 1.2 million new cases of CRC occur annually, accounting for about 10% of all cancers, with a mortality rate of approximately 600,000 [4]. The latest WHO findings cited in GLOBOCAN, is estimated that about 10,96,000 new cases of colorectal cancer will be diagnosed in 2018, while the expected number was 7,04,000. In total, these include 1.8 million new CRC cases [5].
After chemotherapy, more than 50% of patients with CRC relapse, typically with metastasis and 5-year survival of only 10% [6]. In recent years, the pathways for controlling apoptosis, cell proliferation, tumour progression and differentiation, as well as the pathogenic mechanisms of associated processes, have been evaluated to identify the prognostic factors in CRC [7]. Though they are less well understood, epigenetic mechanisms may be as significant as mutations [8]. To date, however, the only reliable prognostic markers in CRC are mutations in BRAF and the RAS family [9,10]. Therefore; clinicians need a better understanding of the biology of CRC and to identify new therapeutic goals [11].
Despite a growing knowledge base, data on the role of EZH2 in CRC are scanty and inconclusive [6] EZH2 is known to be a catalytic unit of the epigenetic regulator of Polycomb Repressive Complex 2 (PRC2) on chromosome 7q35 [12], which affects SUZ12 polycomb repressive complex 2 subunits and the development of embryonic ectoderm necessary for trimethylating histone 3 lysine residue 27 [13,14]. Recent studies have shown that over-expression or mutation of EZH2 occurs in several malignancies [15,16], where it is associated with cell proliferation, invasion, apoptosis, angiogenesis, metastasis, stem cell maintenance, drug resistance, and disease progression; thus, EZH2 is an attractive anti-cancer target [17-20]. Although it has been consistently reported that EZH2 is expressed in CRC, it is unclear whether this expression is positively [21], negatively [22,23], or not associated with patient survival [24]. Also, only one study has considered the role of EZH2 in the growth of CRC cells [24].
This study aimed to study the clinicopathologic significance of EZH2 expression in CRC, the association between EZH2 expression and important clinical variables, and the DFS and OS.
Materials and Methods
This was a retrospective study of primary surgical procedures for CRC performed at Tabriz University of Medical Sciences, Tabriz, Iran, from April 2009 to June 2019. In this study, all participants gave informed consent for the participation in the study and using their Formalin-Fixed Paraffin-Embedded (FFPE) tissue samples. The ethics committee of Tabriz University of Medical Sciences approved the study protocol (permit no 5/d/86136). All patients suspected of colorectal cancer were undergoing colonoscopy. After histological confirmation of colorectal cancer, they were undergoing surgical procedures. All patients in Stage II and III with colorectal cancer treated with FOLFOX 4 or XELOX protocol, as the routine of oncology ward. Also, patients with rectal cancer underwent radiotherapy. Patients with Stage IV diseases checked for KRAS mutations, and in cases with wild type K-RAS, they received anti-EGFR target therapies and chemotherapy too.
Follow-up of the Patients
In non-metastatic colorectal cancer, patients followed every three months for three years, every six months for five years, and then annually. Also, colonoscopy performed for them every three years. In metastatic patients, treatment was continued until the disease progresses, or if the side-effects of the drug were seen. The survival analysis in association of different treatment methods was out of aims of the present study. The OS and DFS were the outcomes of interest. OS was defined as the time from history of surgery to death from CRC, and DFS was defined as the time to recurrence or the last follow-up date.
IHC Analysis
All cases were retrieved from the archived (FFPE) tissue samples (stained with haematoxylin and eosin) and tested them to confirm the pathological diagnosis and grade based on the 2017 World Health Organisation classification of CRC. Tumour size, nodal involvement, metastasis was estimated by (TNM) staging according to the seventh edition of the American Joint Committee on Cancer [25].
Sections for IHC analysis were deparaffinised through graded alcohols and xylene, placed in an EDTA buffer solution (pH 9.0) and then heated to 100°C in a microwave oven at 900 W for 2-5 minutes and 180 W for 5 minutes until boiling. Then, the slides were left in the solution to cool down at room temperature for ~15 minutes and were rinsed in tris-buffered saline (pH=7.6) for 5 minutes. Endogenous peroxidase with 3% hydrogen peroxidase in methanol was added for 10 minutes to block non-specific binding, and then the slides were incubated with the primary antibodies overnight in 4°C. The following primary antibodies were used: EZH2 (AV38470 100 UG; QC: 10904; Sigma) 1.15 diluted phosphate-buffered saline solution. After the slides were incubated in secondary antibody for 30 minutes again incubated in Tris buffer for 5 minutes and incubated with chromogen for 5 minutes. Finally, the slides were washed in Tris buffer saline, prior to sealing a coverslip, counterstained with haematoxylin, and dehydrated with alcohols (96% and 100%) and xylene [2].
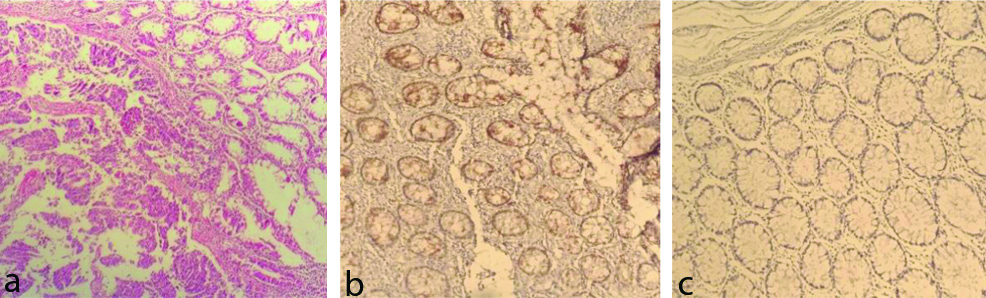
EZH2 immunostaining score was incorporated both staining intensity (0=Negative, 1=Weak, 2=Intermediate, 3=Intensive) and percentage of positive cells (0=0%, 1=>0 -<1%, 2=1-10%, 3=11-33%, 4=34-66%, 5=>67%). The total score of the intensity and the proportion were expressed as the final score. Patients with (range 0-4) expression were combined as the lower expression group, and patients with (range 5) expression were combined as the higher expression group for analyses [Table/Fig-1a-c] [26].
Immunohistochemical analysis of EZH2 in colorectal cancer (100X).
(a) Haemotoxylin and eosin stain; (b) high expression; and (c) low expression.
Paraffin blocks of patients with colorectal cancer were used to evaluate the mutation of KRAS. It was cut to 5 μm for PCR by the Idylla Biocartis NV system (2800 Mechelen, Belgium, BCT006631), in the reference molecular lab, for codons 12, 13, 59, 61, 117, and 146.
Statistical Analysis
The association between EZH2 level (low and high level of EZH2) and clinicopathologic parameters was evaluated by Chi-square test. The Kaplan-Meier method was used to evaluate survival rates and log rank test was used to compare survival of patients with low and high level of EZH2. The uni- and multi-variate Cox proportional hazard regression analysis was performed to evaluated association of EZH2 with OS and PFS, unadjusted and adjusted for confounding variables (Age, Sex, Grade, Stage, EZH2 expression), respectively. The proportional hazards assumption for Cox regression was evaluated based on Schönfeld residuals. To determine the predictive power of EZH2 at the Survival Rate, the Harrell’s C-index was calculated. The p-value <0.05 was considered significant. All statistical analyses were performed in STATA, version 14.
Results
Patients and specimens: Enrolled 100 colorectal cancer patients with mean age of 57.60±13.68 years (range of 21-83 years).
Association of EZH2 expression with clinicopathological aspects: The high EZH2 was expression in 86 (86%) of the patients and Low expression in 14 (14%). There was no association between EZH2 expression and clinicopathologic parameters Also, there was no significant association observed between EZH2 and KRAS, (p=0.92) [Table/Fig-2].
Clinicopathologic characteristics of patients with colon cancer according to the EZH2 level.
| Variables | Total | EZH2 Low (%) High (%) | p-value |
|---|
| Age | <50 | 32 | 6 (18.8) | 26 (81.3) | 0.36 |
| ≥50 | 68 | 8 (11.8) | 60 (88.2) |
| Sex | Female | 41 | 6 (14.6) | 35 (85.4) | 0.80 |
| Male | 59 | 8 (13.6) | 51 (86.4) |
| Site | Ascending | 22 | 3 (13.6) | 19 (86.4) | 0.70 |
| Transverse | 4 | 0 (0) | 4 (100) |
| Descending and rectum | 74 | 11 (14.9) | 63 (85.1) |
| Grade | I | 63 | 7 (11.1) | 56 (88.9) | 0.41 |
| II | 34 | 6 (17.6) | 28 (82.4) |
| III | 3 | 1 (33.3) | 2 (66.7) |
| T | I | 1 | 0 (0) | 1 (100) | 0.90 |
| II | 22 | 4 (18.2) | 18 (81.8) |
| III | 44 | 6 (13.6) | 38 (86.4) |
| IV | 33 | 4 (12.1) | 29 (87.9) |
| N | Positive | 51 | 8 (15.7) | 43 (84.3) | 0.62 |
| Negative | 49 | 6 (12.2) | 43 (87.8) |
| M | Positive | 3 | 3 (13.6) | 13 (86.4) | 0.95 |
| Negative | 11 | 11 (14.1) | 67 (85.9) |
| Stage | I, II | 41 | 6 (14.6) | 35 (85.4) | 0.87 |
| III, IV | 59 | 8 (13.6) | 51 (86.4) |
| KRAS | Wild | 7 | 1 (14.3) | 6 (85.7) | 0.92 |
| Mutant | 5 | 1 (20) | 4 (80) |
| Unknown | 87 | 12 (13.8) | 75 (86.2) |
| DFS | Without relapse | 54 | 7 (13) | 47 (87) | 0.74 |
| Relapse | 46 | 7 (15.2) | 39 (84.8) |
| OS | Alive | 56 | 8 (14.3) | 48 (85.7) | 0.92 |
| Death | 44 | 6 (13.6) | 38 (86.4) |
DFS: Disease free survival; OS: Overall survival; EZH2: Enhancer of zeste homolog 2
Survival analysis by expression of EZH2: The patients who died by the end of the follow-up were 44%. The third quartile of the overall survival distribution (the latest time that at least 75 % of the patients were still alive) was 30.7 days and second quartile (median) of survival time was 73.367 days. Because of low rate of event, the survival curve does not drop to 0.25 or below. So, the first quartile could not be computed. The mean survival time was 80.55 days. There were no significant association between OS and high or low EZH2 expression (log-rank p=0.50; [Table/Fig-3a].
Kaplan-Meier curves showing association of EZH2 expression in patients with colorectal cancer. (a) Overall survival (p=0.50); and (b) Disease-Free Survival (p=0.74).
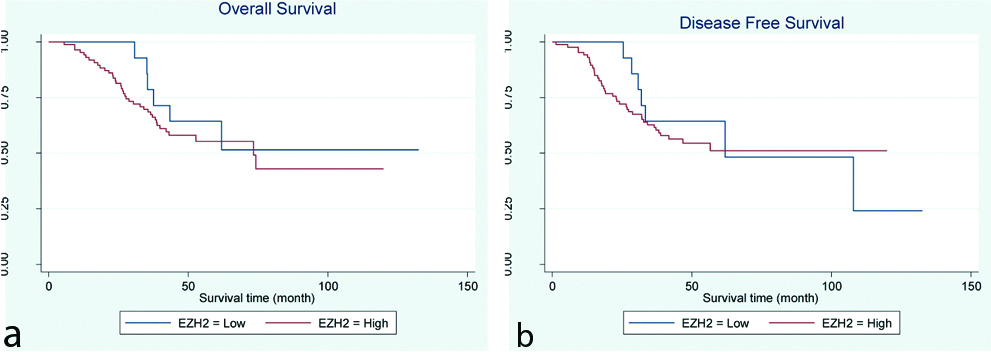
The third quartile of the survival time for DFS (the latest time that at least 75 % of the patients were still alive without any recurrence) was 25.37 days and median time to relapse was 107.83 days. Because of low rate of event, the survival curve does not drop to 0.25 or below. So, the first quartile could not be computed. The mean survival time was 77.81 days. There were no significant association between DFS and high or low EZH2 expression (log-rank p=0.74; [Table/Fig-3b].
Association between patient outcome and EZH2 IHC expression: The proportion hazard assumption was satisfied for OS (p-value=0.19). The Univariate proportional hazard Cox regression analysis showed that OS was not associated with EZH2 expression (HR=1.34, 95% CI=0.57-3.19, p=0.50). Proportional hazard Cox-regression analysis after adjustment for other clinicopathologic parameters showed that OS was not significantly associated with EZH2 (HR=1.53, 95% CI=0.63-3.72, p=0.35). Harrell’s-C index for OS was 0.52.
The proportion hazard assumption was not satisfied with DFS (p-value=0.009). This indicates that the risk ratio of outcome in the two EZH2 groups varied significantly over time. Due to the lack of proportionality assumption, the Cox model with time dependent covariate was processed to the data instead of the conventional Cox model. The time-dependent variable was defined as the interaction between time and the EZH2 variable. After entering the time-dependent variable into the model, the proportionality assumption was established (p-value=0.71). The result of the time dependent Cox model did not show significant effect for the EZH2 variable (HR=7.88 95% CI= .79-78.13, p=.07). In multivariate Cox model for DFS with time dependent variable and adjustment for confounders, the risk level for high EZH2 group was 11.08 times higher than the low EZH2 group (HR=11.08, 95% CI=1.02-119.72, p=0.05).
There were no significant associations between the DFS, OS and other clinicopathologic parameters except for the stage, respectively (HR=3.51, 95% CI=1.71-7.20, p=0.001) (HR=3.29, 95% CI=1.59-6.79, p=0.001) [Table/Fig-4].
Hazard ratios of EZH2 for DFS and OS adjusted for Clinicopathologic characteristics of patients with colon cancer (Multivariate Cox Proportional-Hazards Regression model).
| Variables | DFS HR (95% CI) | p-value* | OSHR (95% CI) | p-value** |
|---|
| Age | <50 | Ref | | Ref | |
| ≥50 | 1.21 (0.60-2.49) | 0.60 | 1.42 (0.70-2.93) | 0.33 |
| Sex | Female | Ref | | Ref | |
| Male | 0.70 (0.40-1.30) | 0.24 | 0.82 (0.44-1.50) | 0.51 |
| Grade | I | Ref | | Ref | |
| II | 0.98 (0.51-1.87) | 0.92 | 1.17 (0.61-2.25) | 0.62 |
| III | 3.85 (0.73-20.12) | 0.11 | 3.24 (0.64-16.30) | 0.15 |
| Stage | I, II | Ref | | Ref | |
| III, IV | 3.55 (1.71-7.35) | 0.001 | 3.30 (1.60-6.80) | 0.001 |
| EZH2 | Low | Ref | | Ref | |
| High | 11.08 (1.02-119.72) | 0.05 | 1.53 (0.63-3.72) | 0.34 |
Lower: Lower bound for 95%; CI: Upper, upper bound for 95%; CI: Adjusted variables: Age, Sex, Grade, Stage
*p-value from time dependent cox regression; **p-value from proportion hazard cox regression
EZH2: Enhancer of zeste homolog 2; DFS: Disease free survival; OS: Overall survival
Discussion
CRC was the third most common cancer in the world in 2013 according to Global Burden of Cancer report [27]. Moreover, the worldwide incidence increased from 1.2 million to 1.7 million cases between 2005 and 2015, representing an overall increase of 36.5% [28,29]. When adjusting for population ageing and growth, even between comparable populations, cases of CRC increased by 5% in Iran over that period, such that it is now the fifth most common cancer [27]. EZH2 is epigenetic regulator of Polycomb Repressive Complex 2 (PRC2) on chromosome 7q35 [12], which is necessary for trimethylating histone 3 lysine residue 27 [13,14]. Recent studies have shown that over-expression or mutation of EZH2 occurs in several malignancies [15,16]. EZH2 plays an important role in the proliferation, invasion, apoptosis, angiogenesis, metastasis, drug resistance, and progression of cancer [2,18,19]. This Oncogene is important to cancer growth through epigenetic silencing of tumour suppressor gene expression [30]. It is known to be over-expressed in prostate cancer [31], lung cancer [32], breast cancer [33], liver cancer [34], and CRC [6] and has a demonstrable association with invasive clinical manifestations [7]. Although incompletely understood, abnormalities of EZH2 and its underlying mechanisms appear to be involved in the pathogenesis of CRC, with a significantly increased expression reported [35].
In this study, the present authors evaluated EZH2 expression in 100 samples of CRC, identifying that EZH2 expression was high in 86 patients and low in only 14 patients. There was no association between EZH2 expression and clinicopathologic parameters. Boostani F et al., reported that high EZH2 expression was associated with clinicopathological features in breast cancer [2]. The present authors could not find any association between any clinicopathological factors and EZH2 expression in CRC. Jiang T et al., reported that high EZH2 expression could be a prognostic factor for both OS and DFS in lung cancer [14]. The present authors also evaluated the association of EZH2 expression with OS and DFS in CRC. EZH2 over-expression was not associated with OS (HR=1.53, 95% CI=0.63-3.72, p=0.35). In association of EZH2 expression with DFS (HR=0.93, 95% CI=0.90-0.10, p=0.05), but considering to the wide confidence interval, this risk ratio needs further investigation.
As has been shown, KRAS is one of the factors indicating prognosis in patients with CRC. Because only 12 tests were available in metastatic patients, survival analysis did not seem statistically significant, so survival analysis was not performed.
In a meta-analysis of studies in Asia, Chen S et al., noticed that EZH2 over-expression was significantly associated with stage and lymph node metastasis, but not with T status [17]. They concluded that it was not possible to confirm the presence of a definite relationship between EZH2 expression and specific carcinoma types because there were too few studies available for anyone type of cancer. In the present study authors didn’t find significant associations between the DFS, OS and clinicopathologic parameters except for the stage, respectively (HR=3.51, 95% CI=1.71-7.20, p=0.001) (HR=3.55, 95% CI=1.71-7.35, p=0.001). Given that Iran is in Asia, conducting further studies with more samples in this population could improve our knowledge of the effect of EZH2 on CRC.
Limitation(s)
There are some limitations that need to be considered with this research. There was a small sample size which could lead to possibility of superiority bias. The study used a shorter follow-up period to analyse survival (which will have made it difficult to assess the effect of EZH2 on disease progression and mortality). The data interpretation was hindered by the lack of an established gold standard for expressing the EZH2 score, which was performed at home in this study. Previous researchers have used different EZH2 ranking methods, so it is possible that this difference in approach accounted for the lack of comparability between data sets. KRAS test were not available for most of the metastatic patients. Despite these limitations, the present study benefits from having evaluated a series of CRCs from a single institute.
Conclusion(s)
In patients with CRC, it was found that EZH2 expression was not associated with clinical features and that it does not appear to be associated with OS or DFS. EZH2 is an attractive target for cancer treatment, but much more research is clearly warranted. Specifically, there is a need to resolve issues with the scoring method and the cut-off threshold for a high EZH2 expression before clinical studies can progress further.
DFS: Disease free survival; OS: Overall survival; EZH2: Enhancer of zeste homolog 2
Lower: Lower bound for 95%; CI: Upper, upper bound for 95%; CI: Adjusted variables: Age, Sex, Grade, Stage
*p-value from time dependent cox regression; **p-value from proportion hazard cox regression
EZH2: Enhancer of zeste homolog 2; DFS: Disease free survival; OS: Overall survival