The term ‘Implant related infection’ is defined as any infection involving the joint prosthesis and adjacent tissue. After redo surgeries (2.1-5.8%) and primary hip or knee replacements (1-2.5%) there has been a variable incidence of implant related infections. Implant related infections though less frequent remains one of the most catastrophic complications associated with increased morbidity and cost [4].
Overall, about 5% internal fixation devices become infected. The occurrence of infection after the internal fixation of closed fractures is 0.5-2% which is generally lower, whereas the incidence may exceed 30% after fixation of open fractures [5]. Orthopaedic implant infections account for about 1% of the total revision arthroplasties while in rheumatoid arthritis patients it could have a higher infection rate of around 3.7% [6].
The most common bacteria that cause prosthesis-related infections among Gram positive agents are Staphylococcus aureus, including MRSA and MSSA, Coagulase Negative Staphylococcus (CoNS) such as S.epidermidis, S.haemolyticus, S.hominis, S.warneri, Enterococcus, Streptococcus viridans. S.aureus and CoNS responsible for approximately half of the infections or more [4]. Among Gram negative agents Escherichia coli, Klebsiella pneumonia, Providencia sp, Serratia marcescens and Citrobacter sp, P. aeruginosa, Acinetobacter sp, are the commonest agents [7,8].
Surgical Site Infection (SSI) is defined as an infection that occurs after surgery in the part of the body where surgery has been taken place. During the first three months post surgery infections are caused by virulent microorganisms such as S. aureus, whereas CoNS are known to cause delayed infections [9].
Because of the complications of prosthetic joint infections, removal of the infected prosthesis and use of intravenous antimicrobial therapy is recommended as it has a mortality rate of 2.7-18% [10]. Consequently, in most cases due to ineffective antibiotic therapy removal of the infected implant is the only option left to cure the infection.
Henceforth, the study aims to evaluate the rate of implant related infections in the institute, the reliability of tissue specimens over wound swabs, the sensitivity of Gram’s stain to identify infections at the earliest and also to understand the current trends of the antibiogram in such infections which would be of better help in initiating appropriate empirical antibiotics.
Materials and Methods
This study was retrospectively reviewed from the samples (tissues and wound swabs) received to the Department of Microbiology at Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India, from October 1st 2017-October 30th 2018 (over a period of one year) in patients with orthopaedic implants.
Microbiological Workup
All the tissues and wound swabs received from patients with post knee and hip replacements, post internal plating, nailing, post Illizarov fixators were analysed. The patients in this study were not on any immunosuppression. Samples were processed as per standard protocols [11]. Each sample was subjected to Gram’s stain, aerobic culture and anaerobic culture. Identification and susceptibility testing was done by using Vitek 2 Compact system. Samples received in sterile containers were only accepted for processing.
Tissue samples were given preference over wound swabs as they are more reliable in diagnostic yield. In superficial infections as it was not possible to take tissues, samples were procured using two sterile swabs, one swab was used for Gram stain and another for aerobic culture and was inoculated on to Blood and chrome agar as per the standard protocols mentioned in Clinical Microbiology Procedures Handbook [11]. MacConkey agar was not used routinely for Gram negative bacilli.
For anaerobic culture the tissue samples were grinded using a homogeniser (tissue grinder) in a reduced medium and was inoculated on blood agar plates and further incubated in anaerobic environment (Gen bag, biomeriux) and placed at 37°C and also into the liquid medium (thioglycollate broth, Hi media) [11].
For aerobic culture the samples were used for Gram stain and inoculated onto chrome agar (CPS, biomeriux) and blood agar (COS, biomeriux). The Gram stain was observed for polymorphs and microorganisms and was reported on the same day. Culture plates were incubated both aerobically and anaerobically at 35-37°C for a minimum of 48 hours. Identification and susceptibility patterns of the bacterial pathogens were identified by the automated Vitek 2 compact system.
MDR organisms are defined as bacteria that are non-susceptible to at least one antimicrobial agent belonging to three or more antimicrobial classes. MDR and ESBL detection was done only by Vitek 2 Compact system. There was no conventional disc diffusion performed for ESBL detection.
Statistical Analysis
The data was analysed in terms of frequency and percentage.
Results
A total of 141 cases were analysed out of which 64/141(45.3%) were tissue samples and 77/141 (54.6%) were wound swabs. The median age of the patients in the study was 40±17.04 years. The total number of males in the study were 108 and females were 33. The number of culture positives observed in tissue samples were 28/64 (43.7%) and in wound swabs was 39/77 (50.6%). Considering the culture positivity, the prevalence of orthopaedic implant related infections was estimated to be around 48.9% [Table/Fig-1]. Mixed infections were observed from 7.1% (2/28) of tissues and 5.1% (2/39) of wound swabs. The percentage of colonisers from wound swabs were observed to be 14.2% (11/77) which showed growth of CoNS and diptheroids [Table/Fig-2].
Analysis of total specimens.
| Sl. No. | Total samples (n=141) | Steriles (n=63) | Culture positives (Including mixed infections) (n=67) 47.5% | Colonisers (n=11) |
|---|
| Tissues | 64 | 36 | 28 | Nil |
| (45.3%) | (56.2%) | (43.7%) | - |
| Wound swabs | 77 | 27 | 39 | 11 |
| (54.6%) | (35.06%) | (50.6%) | (14.2%) |
Type of organisms in culture positive sample.
| Total number of samples (n-141) | Sterile (63) | Monomicrobial (63) | Mixed infection (among culture positives) (4) | Colonisers (11) |
|---|
| Tissue (64) | 36 (56.2%) | 40.6% (26) | 7.1% (2/28) | 0% |
| Wound swabs (77) | 27 (35.06%) | 48.5% (37) | 5.1% (2/39) | 14.2% (11/77) |
Among tissues the percentage of isolation of Gram negative pathogens was higher than the Gram positive pathogens. Escherichia coli (21.4%,6/28) was the most common organism isolated followed by MSSA (17.8%,5/28). Among wound swabs the percentage of isolation of Gram positive pathogens was higher than Gram negative pathogens. MRSA was the most common pathogen isolated from 33.3% (13/39) of wound swabs followed by Escherichia coli (28.2%,11/39) [Table/Fig-3].
Distribution of pathogens in tissues and wound swabs.
| Type of sample (Total No.) | Culture positives | Sterile | Colonisers | Gram positive pathogens | Gram negative pathogens | Mixed infections |
|---|
| Tissues (64) | 28 (43.7%) | 34 (53.1%) | Nil | MRSA-03 (10%) | Escherichia coli-06 (21.4%) | 02/28 (7.1%) |
| MSSA-05 (17.8%) | Enterobacter cloacae-03 (10%) |
| Enterococcus faecalis-01 (3.5%) | Acinetobacter baumannii-03 (10.7%) |
| Streptococcus pyogenes-02 (7.1%) | Pseudomonas aeruginosa-03 (10.7%) |
| Wound swabs (77) | 39 (50.6%) | 27 (35.0%)25 (32.4%) | 11 (14.2%) | MRSA-13 (33.3%) | Escherichia coli-11 (28.2%) | 02/39 (5.12%) |
| Proteus sp-02 (5.12%) |
| MSSA-01 (2.56%) | Acinetobacter baumannii-03 (7.69%) |
| Enterococcus faecium-01 (2.56%) | Pseudomonas aeruginosa-04 (10.2%) |
| MR CoNS-01 (2.56%) | Burkholderia cepacia-01 (2.56%) |
MDR organisms were observed in 25% (7/28) of the tissue isolates and 23% (9/39) of wound swabs. The percentage of ESBL producers in tissue samples were 3.5% (1/28) while from wound swabs it was 10.2% (4/39). Majority of isolates in the study were resistant to penicillins and cephalosporins. The Gram negative isolates in the present study were susceptible to colistin (100%), tigecycline (63.8%), meropenem (55.5%) and piperacillin tazobactam (33.3%) [Table/Fig-4] and among Gram positive isolates 100% susceptibility was observed for vancomycin and teicoplanin [Table/Fig-5].
Antibiotic susceptibility table for Gram negative organisms.
| Antibiotic (Total no. org Gram negative organisms-36) | Susceptible-% (n) | Resistant-% (n) |
|---|
| Amoxicillin | 41.6% (15) | 58.3% (21) |
| Ceftazidime | 41.6% (15) | 58.3% (21) |
| Cefuroxime | 41.6% (15) | 58.3% (21) |
| Ceftriaxone | 41.6% (15) | 58.3% (21) |
| Cefepime | 41.6% (15) | 58.3% (21) |
| Ticarcillin/clavulanic acid | 50.0% (18) | 50.0% (18) |
| Piperacillin/tazobactam | 33.3% (12) | 66.6% (24) |
| Cefaperazone/sulbactam | 38.8% (14) | 61.1% (22) |
| Aztreonam | 36.1% (13) | 63.8% (23) |
| Doripenem | 55.5% (20) | 44.4% (16) |
| Imipenem | 55.5% (20) | 44.4% (16) |
| Meropenem | 55.5% (20) | 44.4% (16) |
| Amikacin | 52.7% (19) | 47.2% (17) |
| Gentamicin | 36.1% (13) | 63.8% (23) |
| Ciprofloxacin | 38.8% (14) | 61.1% (22) |
| Levofloxacin | 52.7% (19) | 47.2% (17) |
| Tigecycline | 63.8% (23) | 36.1% (13) |
| Colistin | 100% | Nil |
Antibiotic susceptibility table for Gram positive organisms.
| Antibiotic (Total no. org Gram positive organisms-27) | Susceptible | Resistant |
|---|
| Benzylpenicillin | 3.7% (1) | 96.2% (26) |
| Oxacillin | 22.2% (6) | 59.2% (16) |
| Ciprofloxacin | 55.5% (15) | 44.4% (12) |
| Levofloxacin | 59.2% (16) | 40.7% (11) |
| Erythromycin | 55.5% (15) | 44.4% (12) |
| Linezolid | 70.3% (19) | 29.6 (8) |
| Daptomycin | 92.5% (25) | 7.4% (2) |
| Teicoplanin | 100% | Nil |
| Vancomycin | 100% | Nil |
| Tetracycline | 66.6% (18) | 33.3% (9) |
Specificity of Gram stain in comparison with tissue cultures in estimating the implant related infections was found to be 83.3% with a sensitivity of 50%.
In the present study the most common risk factors associated with implant infections were diabetes mellitus followed by advanced age, smoking and alcoholism [Table/Fig-6].
Risk factors associated with implant related infections.
| Risk factors | No. of patients |
|---|
| Diabetes mellitus | 59.7% (40/67) |
| Smokers | 11.9% (8/67) |
| Alchoholics | 13.4% (9/67) |
| Advanced age | 17.9% (12/67) |
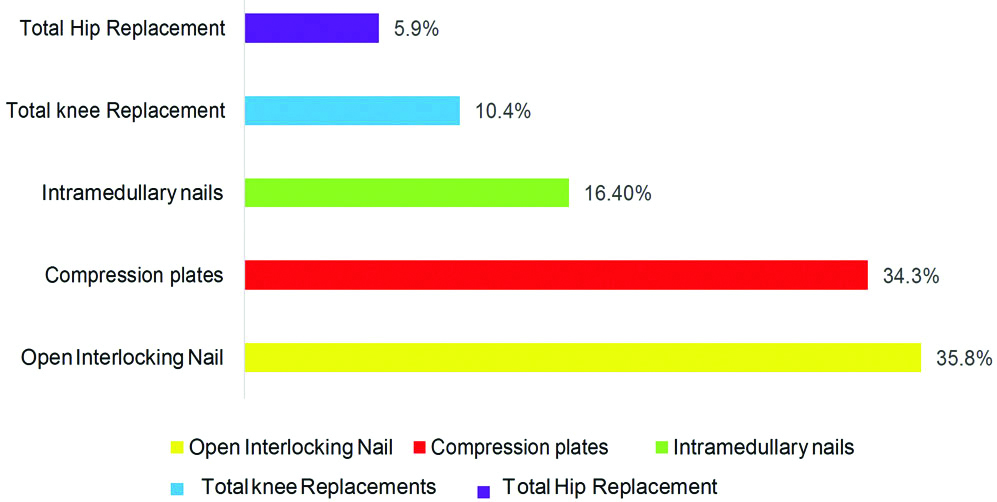
The most common implants which got infected were Open interlocking nails, Compression plate, Intramedullary nails, Total knee implants and Total hip implants [Table/Fig-7]. The most common bone affected was Femur in 22 patients (32.8%), Tibia 16 (23.8%), followed by Radius and Ulna in 6 (8.9%) and 4 (5.9%) patients.
Type of implant most commonly infected.
Discussion
Orthopaedic implants have influenced the treatment of bone fractures and non-infectious arthritis. Orthopaedic implant devices (such as prosthesis for hip, knee, ankle, shoulder and elbow joints and fracture fixation devices such as wires, pins, plates and screws) aim to restore the function of load-bearing joints which are subjected to high levels of mechanical stress, wear and fatigue in the course of normal activity [12]. Infection of these implants may pose a great impediment not only to the patients but also to the surgeons and the community in terms of morbidity and cost due to increased antibiotic use, prolonged hospital stay and repeated debridements [13].
Implant related infections continue to remain a problem for the orthopaedicians. Increase in the number of MDR bacteria emphasises the value of an adequate diagnosis, leading to a proper therapy of these patients. Bacteria have a propensity to adhere to foreign materials like implant surfaces leading to formation of biofilms which further complicates the course of treatment by forming a barrier around these pathogenic organisms by making them more virulent and persistent in causing infections [8].
In the present study there was a preponderance of males over females in a ratio of 3:1, with a median age of 40±17.4 years, similar findings were also observed in the study conducted by Fernandes A and Dias M in which there was a male preponderance (76%) with an average age of 37.1 years [14]. Many risk factors for prosthetic joint infection, such as rheumatoid arthritis, psoriasis, immunosuppression, steroid therapy, poor nutritional status, obesity, diabetes mellitus and extremely advanced age have been reported in various studies [13,15].
Advanced age remains a major contributor in increasing the risk of infections in such patients due to compromised immune response and nutritional status in comparison to the younger population [15]. In the present study a higher percentage of aged patients (17.9%, 12/67) was observed than in the study conducted by Fernandes A and Dias M (8.09%) [14].
Obesity has been associated with an increased risk of infection in many patients. Possible reasons for the increased risk include prolonged operative duration and the presence of other comorbidities [15]. In the present study obesity was not documented however smoking and alcoholism was an important risk factor in 11.9% (8/67) and 13.4% (9/67) of patients respectively which was similar to the study done by Pradella JGDP et al., [16].
Diabetes mellitus has also been associated with an increased risk of Prosthetic Joint Infection (PJI). Perioperative hyperglycemia may cause disruption of the host response to a bacterial load. This may be due to increased biofilm formation in the presence of elevated levels of glucose, impaired leukocyte function or microvascular changes in patients with diabetes, which may influence wound healing and the development of infections [15]. Majority of the cases in the present study had diabetes as the predisposing factor (59.7%,40/67) which was very high compared to the study done by Fernandes A and Dias M (8.09%) [14].
Chronic conditions like Rheumatoid arthritis, malignancy and patients on immunosuppressive medications have shown to have a higher risk of PJI. The incidence of infection following arthroplasty revision surgery is higher than that following primary implantation [15]. In the present study no case of Rheumatoid arthritis with implant infection was documented.
In a meta-analysis by Tande AJ and Patel R of 14 large studies including >2400 patients with arthroplasty infection it was observed that majority of the infections were caused by Gram positive cocci out of which 50% were caused by S.aureus, <10% caused by Enterococcus and Streptococcus sp. whereas aerobic Gram-negative bacilli were involved in <10% of cases of knee and hip prosthetic joint infection [17]. A similar finding was also observed in the present study.
In a study conducted by Fernandes A and Dias M, the most common sites affected were the femur (26%), tibia (16%), bimalleolar (16%) and the humerus (8%), followed by the radius, the ulna and the tarsal bones which correlated well with the present study in which Femur was the most commonly affected site (30.9%,22) followed by Tibia (22.5%,16), Radius (8.45%,6) and Humerus (5.6%,4) [14].
One of the most debilitating complication of arthroplasty is PJI. In a study conducted by Fourcade C et al., the most common organism isolated was Coagulase- negative Staphylococcus (n=14), Staphylococcus aureus (n=10) and members of Enterobacteriaceae (n=5) [18].
Lakshmi Narayana SA et al., showed that Methicillin Resistant Staphylococcus aureus was the most commonly isolated pathogen [5]. While in the present study also it was observed that both Methicillin Resistant Staphylococcus aureus and Escherichia coli were the most common isolate.
In the present study Staphylococcus aureus was the most common cause of implant related infection 28.5% in tissues and 35.8% in wound swabs which was similar with the study conducted by Berbari EF et al., in which Staphylococcus aureus (28%) was one of the most common isolate [19].
Bongartz et al., observed that maximum number of infections were caused by Staphylococcus aureus accounting for 65.2%, Streptococcus sp. 17.4%, Gram-negative bacilli 8.7% of cases [20].
In the study conducted by Lakshmi Narayana SA et al., all the MRSA isolates were susceptible to vancomycin and teicoplanin which was also observed in the present study, while for Gram negative isolates a high percentage of resistance was observed in the present study [5].
In the study conducted by Bongartz T et al., in Rheumatoid arthritis patients the most common joint infected was knee (10.4%,7/67) followed by hip (5.9%,4/67) with a less percentage [20].
Though anaerobes play a significant role in the pathogenesis of implant infections, no anaerobes were isolated in this study. In the study conducted by Fernandes A and Dias M among positive cultures, 35.7% showed a mixed culture of more than two organisms where as in the present study mixed cultures in tissue samples were observed in only two cases of compound fractures following road traffic accident [14]. In the above study the prevalence of ESBL was 31.7% while in the present study the prevalence of ESBL was much less 7.4% (5/67) this might be due to a higher percentage of MDR organisms (23.8%,16/67) and in the above study the prevalence of MRSA was 12.7% while in the present study it was much higher (23.8%,16/67).
Sonication is one of the valuable technique for improving the culture positivity in implant related infections, in studies conducted by Fernandes A and Dias M et al., and Khosravi AD et al., showed a high culture positivity of 84% and 94% respectively whereas in the present study the rate of culture positivity was very less (47.5%) compared to the above studies, probably as sonication of implants has not been done as a routine procedure [14,21].
For the standard care of postoperative infections repeated surgeries, debridement and substitution of implants are required [22]. The complications associated with these longstanding and recurrent infections causes impairment of function, disability, amputation and death of the patient [23,24]. Hence the best treatment strategy is prevention of such devastating implant related infections.
Antimicrobial prophylaxis remains the most effective method of reducing the prevalence of implant infections [25]. In all the cases of present study with implant infections, cefazolin was preferred as prophylaxis and had a successful outcome. Patients were treated with IV antibiotics cloxacillin Intravenous (IV) 1 gm 6th hourly against MSSA isolates and IV vancomycin 15 mg/kg 12th hourly was the drug of choice against MRSA isolates. All the ESBL producers were treated with B-lactamase inhibitor combinations like IV pip taz 4.5 gms 8th hourly and the MDR organisms were treated with dual therapy of IV Colistin 2 mu and IV Meropenem 1 gm 8th hourly.
As the internal fixation implants are considered to be sterile, the most probable source of exposure may be acquired exogenously during trauma or in the perioperative period [9,26], a similar justification also applies for the implant related infections in the present study.
In implant related infections tissue sampling influences the yield of microbiological cultures as bacteria can infect different sites of an implant as well as in peri-implant tissue, henceforth the need for appropriate sampling is essential for isolation of pathogens.
Limitation(s)
As this was a retrospective study, further follow-up of patients could not be done.
Conclusion(s)
In the present study it was observed that perioperative tissue specimens are reliable samples than wound swabs, as most of the superficial wound swabs are colonised with skin flora and chances of isolation of mixed flora remains high and inconclusive which would delay the diagnosis and prompt initiation of empirical therapy. This study suggests the need for adequate prophylactic antimicrobial therapy to optimise the chances of treatment success and prevent postoperative infections. The choice of empiric antibiotics should be based on the local antibiogram and a good protocol should be formulated for prevention of orthopaedic implant related infections. Proper infection control practices should be followed to prevent financial burden on the patient as well as on the hospital resources.