Prostate cancer and BPH are the two most common forms of prostate tumour. Both are found predominantly in elderly males. Three quarter of the prostate cancer cases occur in men above 65 years of age with peak age of incidence between 70 and 74 years [1]. The disease is therefore more common in countries with higher proportions of elderly men in their population. Incidence of prostate cancer in India is lower than that in the Western populations. However, according to the National Health Profile 2019 [2], life expectancy in India has increased from 49.7 years in 1970-75 to 68.7 years in 2012-2016. This shift in the demographic picture has set the stage for an increased burden of diseases, specific to the elderly population. Incidence of prostate cancer as reported by Chatterjee A from a study in eastern India, revealed a drastic increase from 5.71% in 2003-2006 to 17.76% in 2007 and 28.97% in 2010 [3]. Most early stage prostate cancers are asymptomatic and difficult to detect clinically. The disease either becomes locally advanced or metastatic.
Aeiopathogenesis of prostate cancer includes, among various factors, oxidative stress and one carbon metabolism. The prooxidant-antioxidant balance of prostate cancer tissue is shifted towards a state of oxidative stress when compared to normal prostate tissue [4]. The existence of oxidative stress in prostate cancer is further supported by the beneficial role of various antioxidant trials [5]. Not only that, study had also shown that Reactive Oxygen Species (ROS) generation is essential for invasive phenotype of prostate cancer [6]. One carbon metabolism, on the other hand, has been linked to tumourigenesis through two principle pathways of DNA methylation and DNA synthesis and repair. Methylation is a well-known epigenetic mechanism to silence gene expression. Methylation of CpG islands in the promoter region of genes may be especially pertinent in the prostate cancer development [7]. In addition, several other genes including tumour suppressors PTEN/MMAC1 and CDKN2, have been observed to be hyper methylated and inactivated in prostate cancer [7].
Keeping above background, parameters like MDA (for oxidative stress), Vitamin B12 and homocysteine (for status of the one carbon metabolism) were selected for the present study to investigate some of the multifactorial aetiology of prostate neoplasms. Study on these parameters in prostate neoplasms are very few in number and the results are also far from any definite conclusion. Hence, present study aims to compare serum level of these parameters between control, BPH and prostate cancer cases to find any association.
Materials and Methods
Study Population
A cross-sectional observational study was conducted among patients either attending the outdoor or admitted in the in-patient Department of Urology and were scheduled for undergoing Trans-Rectal Ultrasound (TRUS) guided biopsy because of abnormally high Prostate Specific Antigen (PSA) value or Digital Rectal Examination (DRE) findings. The study was conducted during a time period of January 2018 to June 2019. Ethical clearance (IPGME&R/IEC/2018/313) was duly obtained from the Institutional Ethics Committee of Institute of Post-graduate Medical Education and Research, Kolkata, West Bengal, India. Informed written consent was taken from each patient.
After exclusion of 40 patients based on exclusion criteria (female patients, histologically unconfirmed cases of BHP or prostrate cancer, or the patients already receiving anti-cancer therapy or have already undergone surgical procedure for Ca prostrate, patients with co-morbid conditions (eg. diabetes, obesity, dyslipidemia, hypertension, other cancers, liver diseases, renal failure), patients on anti-oxidants or multi-vitamin medications, or those who have altered PSA level for any other prostrate pathology like prostatitis etc.), 80 subjects were selected as case for the study on the basis of predetermined inclusion and exclusion criteria in age group of 50-90 years and biopsy report of each case was collected from the pathology department. Out of these 80 cases, 40 patients had histopathologically confirmed BPH and 40 patients had histopathologically confirmed prostate cancer and they were labelled as two separate case group. Forty more subjects, who were apparently healthy and age matched, were chosen from the urology outpatient department as the control group.
Sampling Technique
A 5 mL of venous blood sample was drawn from a large peripheral vein of each subject in EDTA vial following strict aseptic precautions. Serum was separated by centrifugation (3500 rpm for 5 minutes) and stored at -20°c. All groups were assessed for MDA (by TBARS assay) and PSA, vitamin B12 and homocysteine (by chemiluminescent method). TBARS assay [8] is based on the acid catalysed decomposition of lipid hydroperoxides to MDA that reacts with Thiobarbituric Acid (TBA) to form a pink coloured chromogen, which is then evaluated spectrophotometrically at 532 nm. Vitamin B12, homocysteine (Hcy) and PSA were analysed by Chemiluminescent Immune Assay (CLIA) method (IMMULITE 1000Homocysteine, Vitamin B12 and PSA kit, SIEMENS). Biochemical parameters such as fasting and post-prandial blood sugar, urea, creatinine and liver function tests were also measured (by spectrophotometric method) to rule out diabetes and any underlying hepatic and renal pathology which might have affected the study parameters if present.
Statistical Analysis
Data were analysed by software-Statistical Package for the Social Sciences (SPSS)-version 24. All variables are normally distributed by Kolmogorov-Smirnov goodness-of-fit test and the comparison between cases and controls were done by student’s unpaired t-test. Analysis was two tailed and p<0.05 was considered statistically significant.
Results
In the present study, 120 subjects (80 cases and 40 control) were included. Mean age of control, BPH and Prostate cancer cases was 67.83±7.3, 69.55±7.6 and 66.00±9.4 years, respectively [Table/Fig-1,2].
Comparing means of statistical data between control and BPH cases.
| Parameters | Control subjects (n=40) Mean±SD | BPH cases (n=40) Mean±SD | p-value* |
|---|
| Age (years) | 67.83±7.296 | 69.55±7.649 | 0.305 |
| PSA (ng/mL) | 1.471±0.584 | 5.98±2.45 | <0.0001 |
| Hcy (μmol/L) | 9.51±3.03 | 27.9±4.86 | <0.0001 |
| Vitamin B12 (ng/mL) | 283.9±90.4 | 294.7±32.2 | 0.477 |
| MDA (nmol/mL) | 3.23±1.53 | 5.58±2.02 | <0.0001 |
| FBS (mg/dL) | 93.93±7.43 | 95.9±4.21 | 0.637 |
| PPBS (mg/dL) | 131.55±5.8 | 130.46±2.45 | 0.452 |
| Urea (mg/dL) | 28.03±11.57 | 28.50±4.25 | 0.808 |
| Creatinine (mg/dL) | 0.95±0.123 | 0.914±0.127 | 0.207 |
| Total bilirubin (mg/dL) | 0.750±0.179 | 0.783±0.211 | 0.459 |
| Albumin (g/dL) | 4.19±0.219 | 5.12±1.24 | 0.598 |
| SGPT (U/L) | 30.43±3.01 | 31.2±0.705 | 0.246 |
*p-value calculated by student’s unpaired t-test; PSA: Prostate specific antigen; Hcy: Homocysteine; MDA: Malondialdehyde; FBS: Fasting blood sugar; PPBS: Post prandial blood sugar; SGPT: Serum glutamic pyruvic transaminase
Comparing means of statistical data between control and prostate cancer cases.
| Parameters | Control subjects (n=40) Mean±SD | Prostate cancer (n=40) Mean±SD | p-value* |
|---|
| Age (years) | 67.83±7.296 | 66.00±9.430 | 0.336 |
| PSA (ng/mL) | 1.471±0.584 | 42.9±10.7 | <0.0001 |
| Hcy (μmol/L) | 9.51±3.03 | 54.2±11.9 | <0.0001 |
| Vitamin B12 (ng/mL) | 283.9±90.4 | 314.4±68.7 | 0.094 |
| MDA (nmol/mL) | 3.23±1.53 | 35.8±9.45 | <0.0001 |
| FBS (mg/dL) | 93.93±7.43 | 97.5±6.99 | 0.437 |
| PPBS (mg/dL) | 131.55±5.8 | 135.7±5.24 | 0.342 |
| Urea (mg/dL) | 28.03±11.57 | 27.7±5.52 | 0.871 |
| Creatinine (mg/dL) | 0.95±0.123 | 0.957±0.043 | 0.718 |
| Total bilirubin (mg/dL) | 0.750±0.179 | 0.755±0.179 | 0.559 |
| Total protein | 7.12±0.249 | 7.085±0.267 | 0.492 |
| Albumin (g/dL) | 4.19±0.219 | 4.44±0.678 | 0.669 |
| SGPT (U/L) | 30.43±3.01 | 33.2±3.12 | 0.14 |
*p-value calculated by student’s unpaired t-test; PSA- Prostate specific antigen; Hcy: Homocysteine; MDA: Malondialdehyde; FBS: Fasting blood sugar; PPBS: Post prandial blood sugar; SGPT: Serum glutamic pyruvic transaminase
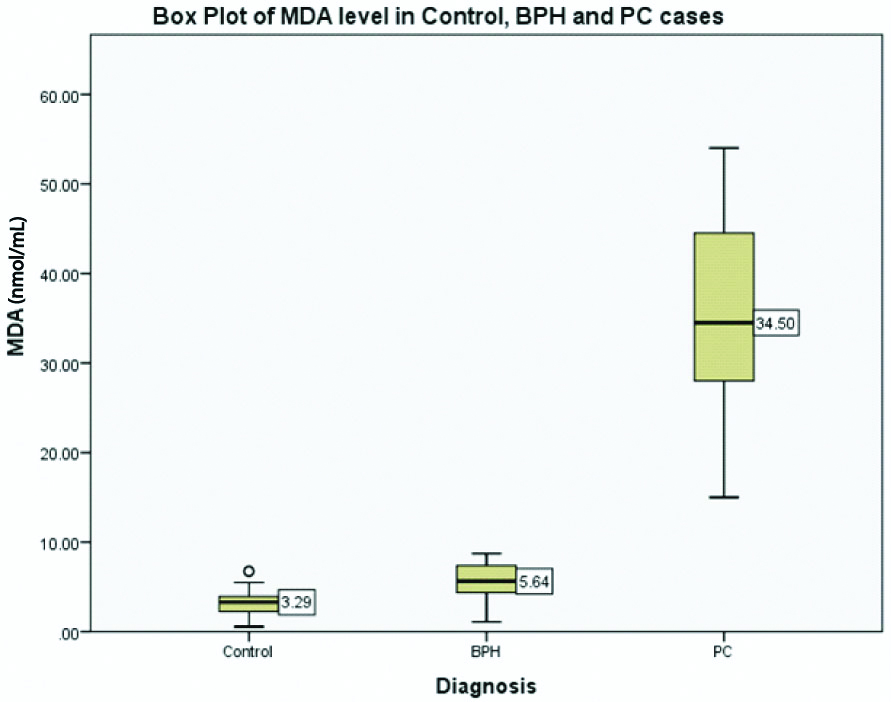
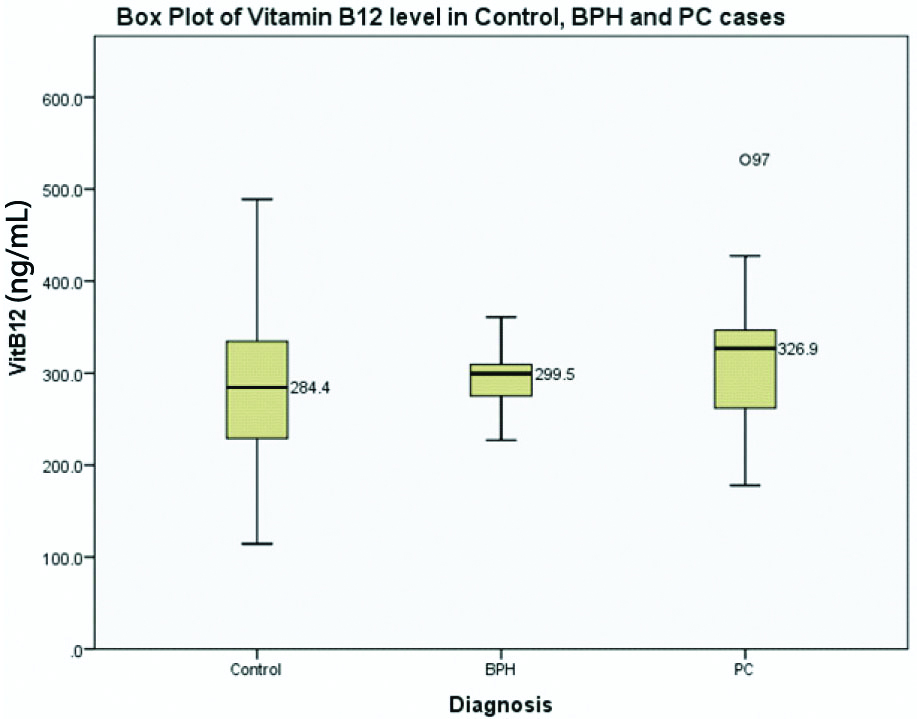
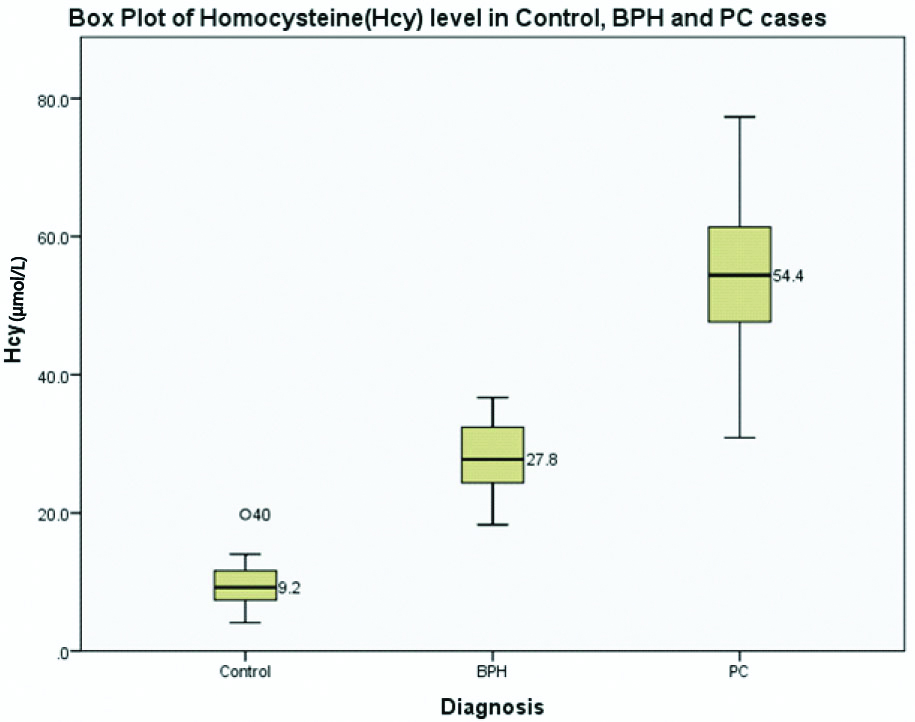
When means were compared (by student’s unpaired t-test) between control and BPH cases, difference of MDA and Hcy was found statistically significant (p<0.0001 for both MDA and Hcy), whereas no significant difference of vitamin B12 (p=0.477) was found. Comparison of other parameters was not significant [Table/Fig-1]. Similarly, between control and prostate cancer group, there was statistically significant difference for MDA and Hcy (p<0.0001) but difference for vitamin B12 was insignificant (p=0.094). For rest of the parameters, comparison was statistically not significant [Table/Fig-2]. The above comparisons were also demonstrated in box plot [Table/Fig-3,4 and 5].
Comparison of MDA between control, BPH and PC case.
Comparison of vitamin B12 Between control, BPH and PC case.
Comparison of HCY between control, BPH and PC case.
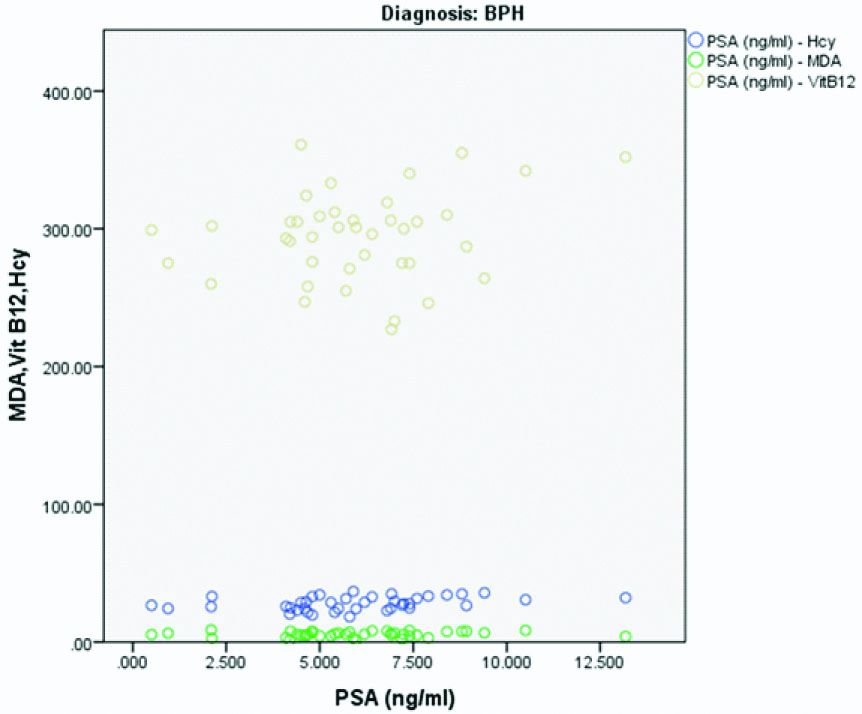
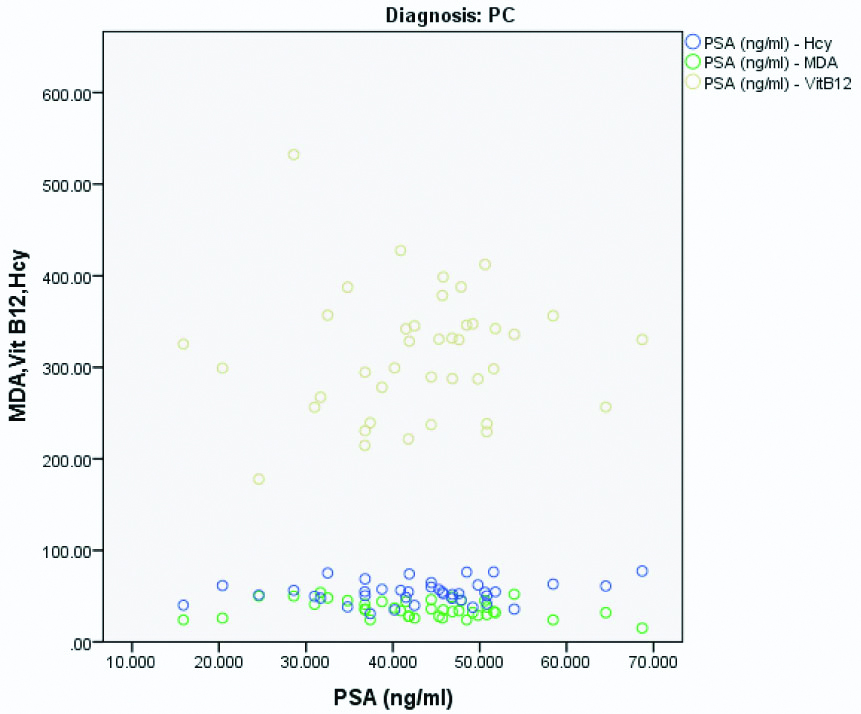
Variables were compared between them in BPH and prostate cancer separately and Pearson’s correlation coefficient was calculated. In BPH, correlation between PSA and MDA showed a positive correlation (correlation coefficient r=0.122, p-value=0.455). Similar positive correlation was also found in BPH between PSA and Hcy (r=0.368, p=0.020) and between PSA and Vitamin B12 (r=0.217, p-value=0.179) [Table/Fig-6]. On the other hand, in prostate cancer, a positive correlation was found between PSA and Vitamin B12 (r=0.063, p-value=0.697), as well as between PSA and Hcy (r=0.226, p=0.160). However, correlation between PSA and MDA in Prostate cancer was negative (r=-0.282, p-value=0.078) [Table/Fig-7].
Scatter diagram showing correlation between MDA, Vitamin B12, HCy and PSA in BPH.
Scatter diagram showing correlation between MDA, Vitamin B12, HCy and PSA in prostate cancer.
Discussion
Aetiology of prostate neoplasms is multifactorial. Epidemiological studies have implicated a range of possible aetiological factors (such as high saturated fat intake, low intake of carotenoids, vasectomy and other sexual factors, high serum androgen levels [4], family history of prostate cancer, and increased generation of ROS) responsible for the development of the above-mentioned diseases. In the present study some representative biochemical parameters among the causative agents had been studied in patients of prostate neoplasm and compared with the control group.
The MDA level, measured as a marker of oxidative stress was found significantly increased in BPH and prostate cancer patients when compared to control group. Oxidative stress develops when there is excess of free radical generation and/or decreased activity of antioxidant system. In prostate cancer, both of these mechanisms may contribute to a state of oxidative stress. Increased generation of free radicals in prostate cancer may be due to dietary fat consumption, androgen exposure or inflammation in prostate tumour tissue. Whereas decreased antioxidant activity such as lowered intracellular glutathione reserve and decreased activity of some antioxidant enzyme systems (e.g., gamma glutamyl transpeptidase) may also be caused by androgen exposure [4]. According to one model of prostate carcinogenesis, chronic infection leads to chronic inflammation, and defects in antioxidant enzymes, DNA repair mechanisms, and apoptosis, which later combine to initiate carcinogenesis. Oxidative stress cause DNA damage in the form of strand breakage, base modifications, DNA protein cross linkages that in turn lead to further mutation, chromosomal aberration, genomic instability and eventually cancer. Activation of transcription factors (e.g., NF-κB) by oxidative stress alter intracellular signal transduction system to induce proto-oncogenes such as c-fos, c-myc oncogenes and cause p 53 mutation [9] bringing about the progression of cancer. On the other hand, free radical mediated oxidative stress plays an important role in the pathogenesis of BPH. Studies have shown higher level of oxidative stress marker such as 8-OH deoxyguanosine (8-OH dG) [10] and decreased level of antioxidant enzymes such as Superoxide Dismutase (SOD), glutathione peroxidase in BPH. From the results of the present study, analysis for oxidative stress markers may be proposed and provision of antioxidant medicine supplementation along with the conventional treatment protocol might also be considered for these patients. One carbon determinant has been reported to be involved in cancer development, but results vary according to the tissue of origin of the malignancy [11].
In the present study, the members related to one carbon metabolism like vitamin B12, and homocysteine were measured. The enzyme methionine synthase requires Vit B12 as coenzyme and the reaction also involves recycling of tetrahydrofolate which may be utilised for nucleotide biosynthesis. That is why, cancer patients are expected to have low plasma folate because tumour cells, due to high rate of cell division utilise folate from circulation to synthesise DNA (de-novo purine synthesis). On the other hand, relatively high folate status is generally known to reduce risk of cancer development. This protective effect of folate level was documented in colon cancer [11]. However, based on the above fact, supplementation of folate has not been beneficial in cancer patients [12], rather it has accentuated advanced lesion in the folate supplemented groups when compared to placebo arm. Vitamin B12, a closely related compound to folate metabolism was measured in the BPH and prostate cancer patients. The level of serum vitamin B12 was 283.9±90.4 in control subjects vs (314.4±68.7, p>0.05) in prostate cancer patients and (294±32.2, p>0.05) in BPH patients. Thus, the difference has no statistical significance. These results were in accordance with a nested control study by Weinstein SJ et al., and Johansson M et al., which found no significant association for circulating Vitamin B12 with prostate cancer risk [13,14]. The unequivocal results may partially originate from the dual effect of folate in carcinogenesis. Epigenetic events affect gene expression without altering the actual sequence of DNA. Examples include DNA hypomethylation (may involve Vit B12), chromatin remodeling, histone modification, and RNA interference. Other than methylation, folate by promoting synthesis of thymidylate from uracil exerts its protective effect (may not be dependent on Vit B 12). Mis-incorporation of uracil in DNA increases genomic instability and leads to cancer [15]. Another study by Collin SM et al., found positive association of increased serum vitamin B12 concentration with prostate cancer risk (highest versus lowest quartile of B12 Odds Ratio (OR)=1.17 (95% confidence interval, 0.95-1.43) [16]. Interestingly, hypermethylation of CpG island sequences of the Glutathione S-Transferase k gene (GSTP1) is a frequently reported epigenetic change in prostate cancer [7], which may require increased activity of Vitamin B 12 as coenzyme. Thus, the results of studies on the associations of circulating vitamin B12 and risk of prostate cancer are highly varied and far from any definite conclusion yet.
In this study, Hcy was also measured in all three groups. Serum Hcy level was 2.93-folds higher in BPH cases and 5.69 folds higher in prostate cancer cases compared to control group. Association of increased Hcy levels have been seldom reported in scientific literature. Study by Hultdin J et al., found increasing plasma Hcy levels associated with a reduced risk of borderline significance (OR=0.67; 95% CI=0.431.04; Ptrend=0.08) [17]. However, after adjustment for other 2 plasma variables, BMI and smoking, the association becomes statistically non-significant (adjusted OR of 0.91 (95% CI=0.51-1.58; Ptrend=0.60). In ProtecT study, Hcy were not associated with increased prostate cancer risk [16] and a 2003 study of Weinstein SJ et al., reported no association with prostate cancer risk for circulating concentrations of Hcy [13]. Global DNA hypomethylation is a common finding in prostate cancer. This epigenetic change is carried out by the enzyme methyl transferase which utilises S-adenosyl methionine or SAM as methyl donor. The SAM-to-SAH ratio determines cellular methylation potential and in hyper homocysteinemic state, this ratio decreases leading to decreased methylation potential [18].
Limitation(s)
The sample size was too small to generalise conclusions of test on other populations and thus raises concerns about type 1 error for significant findings and type 2 error for our null associations. Second limitation was the observational, cross-sectional design used in present study could only assess association, but could not confirm causal relationship for which a prospective study is needed. Thirdly, alcohol consumption, as well as drugs and gastrointestinal disorders interfering with folate or vitamin B12 bioavailability or uptake, such as antacids or certain anticonvulsants and gastritis or celiac disease, was also not addressed in the present study. Fourthly, association of parameters in localised vs advanced stage prostate cancer was not considered, which might have revealed more insight into the pathogenesis of prostate cancer and role of one carbon metabolites in it.
Conclusion(s)
The present study suggests that high level of oxidative stress is associated with prostate cancer and BPH cases compared to age matched control group. Possible role of antioxidant medications in modulating outcome in these patients, however needs exploration by randomised trials. In addition, it was also found that there was an association of high Hcy level and tumours of prostate origin whereas with vitamin B12, no association was documented. Further, longitudinal studies are required to establish any causal relationship. Association of hyper homocysteinemia with BPH and prostate cancer, if proved by larger prospective studies, may indicate the emergence of Hcy as a novel tumour marker in conjunction with PSA.
*p-value calculated by student’s unpaired t-test; PSA: Prostate specific antigen; Hcy: Homocysteine; MDA: Malondialdehyde; FBS: Fasting blood sugar; PPBS: Post prandial blood sugar; SGPT: Serum glutamic pyruvic transaminase
*p-value calculated by student’s unpaired t-test; PSA- Prostate specific antigen; Hcy: Homocysteine; MDA: Malondialdehyde; FBS: Fasting blood sugar; PPBS: Post prandial blood sugar; SGPT: Serum glutamic pyruvic transaminase