Endoscopic stent placement is an eminent procedure in urological practice and it substantially helps in reducing severe symptoms of ureteral obstruction. However, there may be few adverse events which may significantly affect the QoL of patients. These include stent related pain, sexual dysfunction, haematuria, dysuria, etc., [1-3].
A meta-analysis suggested that the use of alpha blockers could be effective in view of the similarity of these symptoms to benign prostatic hyperplasia related LUTS [4]. Various pharmacological adjuncts have been tested, but none of the combinations have proven to be definitive in relieving these symptoms considerably.
Silodosin, a highly selective α1-adrenoreceptor antagonist, is generally used for the symptomatic treatment of LUTS through relaxation of smooth muscle cells of the lower urinary tract [5-7]. Deflazacort, a glucocorticoid, is used as an anti-inflammatory and immuno-suppressant. In urology practices, it has been used in medical expulsive therapy for distal ureteric stones due to its anti-edema role [8-11].
In spite of several available studies on use of combination of alfa blockers and deflazacort for relief of stent related symptoms, this study will add evidence to the literature. The primary objective of this study was to evaluate the effect of silodosin and deflazacort combination in improving LUTS. The secondary objective was to evaluate QoL in patients with indwelling DJ ureteral stents.
Materials and Methods
This was a randomised, double-blind, parallel group single center study, conducted at the Department of Urology of a Tertiary Care Hospital in Eastern India, between August 2018 and December 2018. This study protocol was approved by the Institutional Ethics Committee (IEC/DMR/IMS-SOA/2018/017). The study was conducted in accordance with the approved protocol, and ethical principles that have their origin in the Declaration of Helsinki and latest amendment (2013). Each study participants provided written informed consent for participation in the study.
Inclusion criteria: Patients of either sex, aged between 18 and 50 years who were undergoing unilateral Percutaneous Nephrolithotomy (PCNL) or Ureteroscopic Lithotripsy (URSL) with DJ stenting were eligible to participate.
Exclusion criteria: Patients with history of diabetes, hypertension, renal failure or osteoporosis, patients who were receiving nitrate drugs, patients with postoperative residual stone fragments, pregnant or feeding women, patients with bilateral stents, long-term stenting and bladder/prostate pathology were excluded from the study. Patients with history of LUTS, history of chronic use of selective alpha-1 blockers, or history of hypersensitivity to diclofenac, cephalosporins or fluoroquinolones were also excluded from study.
After screening, patients who were eligible and agreed to participate, were randomised (1:1) to one of the treatment groups to receive silodosin 8 mg once daily (Group A) or silodosin 8 mg plus deflazacort 30 mg once daily (Group B) for two weeks. The randomisation codes were generated using online randomisation tool by a resident who was not involved in the study. Once the patient was screened and found eligible, the resident was contacted for randomisation code to enroll the patient. To maintain the blinding, sugar-coated tablets with similar shape and size were used along with silodosin 8 mg for Group A. Analgesics (diclofenac) was allowed on as required basis. Analgesic requirement and adverse events during study period were noted.
The outcome measures included pain and voiding flank pain which were evaluated using 10-cm linear VAS and the irritability with DJ stent was evaluated using the irritative domain of IPSS before DJ stent removal (two weeks after procedure). Scores for frequency, urgency and nocturia were assessed. QoL due to urinary symptoms was assessed on a 6-point scoring system where 0 indicates delightful and 6 indicates terrible [12].
For URSL, 6.5/8.5 Fr Ureteroscope (Wolf) and pneumatic Lithotripter were used; however, for PCNL, 26 Fr Nephroscope (Wolf) and pneumatic Lithotripter were used. The decision to place DJ stents was taken by the operating urologist based on clinical judgement considering stone burden, mucosal trauma and need for ureteral dilation to access tight ureters, and was not specified by the protocol. Postoperative X-ray (KUB) was done in all patients to rule out residual stone fragment. On the day of surgery, as prophylaxis, each patient received ceftazidime 1 gm (intravenously). In all the patients Foley’s catheter was removed on postoperative Day 1. As per institutional protocol, each patient received oral levofloxacin 500 mg once daily for seven days. Patients were informed about DJ stent related symptoms and were given IPSS and VAS at discharge. Patients were instructed to follow-up after one and two weeks or on as required basis.
Based on our clinical experience and limited duration of study (academic project), a sample size of 150 was considered sufficient to evaluate stent related symptoms.
Statistical Analysis
Data was collected and tabulated in an excel sheet and was analysed using IBM SPSS version 20.0 licensed to the institute for generation of inferential statistics. Student’s t-test was used for comparison of VAS and IPSS scores. A p-value of <0.05 was considered significant.
Results
A total of 151 patients were enrolled (Group A, n=75; Group B, n=76). Two patients were lost to follow-up. One patient had urinary tract infection and two patients had haematuria leading to early removal of stent. Two patients had stent migration [Table/Fig-1]. In total, 144 patients were analysed for the study.
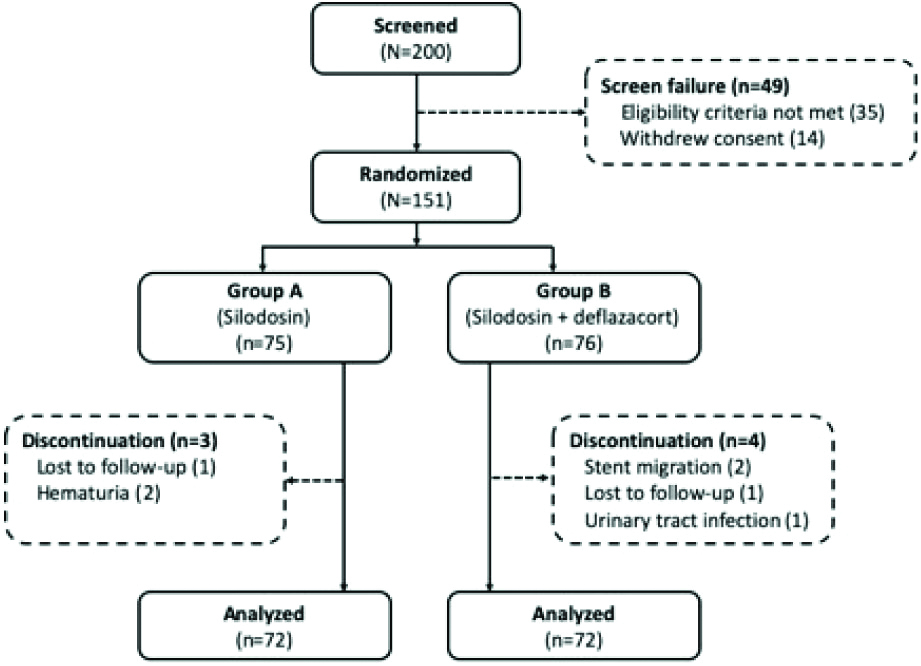
The patient demographics, duration of surgery were comparable between the groups [Table/Fig-2]. The mean age was 50.93 and 50.91 years in Group A and B, respectively (p<0.988); 60 (83.33%) and 58 (80.56%) patients were males respectively. The mean duration of surgery was 28.56 and 29.1 minutes in Group A and B, respectively; however, the mean stone size was 9.45 and 9.24 mm, respectively. A total of 59.72% (n=43) patients from Group A underwent PCNL and 40.28% (n=29) underwent URSL; similarly, 61.11% (n=44) patients from Group B underwent PCNL and 38.89% (n=28) underwent URSL.
| Variables | Group A (n=72) | Group B (n=72) | p-value |
|---|
| Age (years) | 50.93 (10.72) | 50.91 (9.65) | <0.988 |
| Weight (kg) | 66.4 (4.54) | 68.7 (3.11) | 0.640 |
| Sex, n (%) |
| Males | 60 (83.33) | 58 (80.56) | 0.640 |
| Females | 12 (16.67) | 14 (19.44) |
| Duration of surgery (min) | 28.56 (4.68) | 29.1 (4.64) | 0.618 |
| Stone size (mm) | 9.45 (1.62) | 9.24 (1.52) | 0.150 |
| Procedures |
| PCNL | 43 (59.72) | 44 (61.11) | - |
| URSL | 29 (40.28) | 28 (38.89) |
Data is presented as mean (SD), unless otherwise specified; PCNL: Percutaneous nephrolithotomy; SD: Standard deviation; URSL: Ureteroscopic lithotripsy
As shown in [Table/Fig-3], overall, there was a significant (p<0.05) reduction in Group B as compared to group A in both pain and voiding flank pain. The mean score of frequency on the IPSS and urgency were more in Group A than Group B. The mean score of nocturia on the IPSS was 1.06 in Group A and 1.01 in Group B (p=0.87). The mean score of QoL on the IPSS was significantly reduced in group B (1.44) compared to Group A (2.81) (p<0.05). There was no significant difference in the use of diclofenac for pain relief. There were no reported adverse events in any of the groups.
Comparison of ureteral symptom scores and VAS scores.
| Parameters | Group A (n=72) | Group B (n=72) | p-value |
|---|
| Pain (VAS) | 2.01 (0.84) | 1.05 (0.35) | <0.05 |
| Voiding flank pain (VAS) | 2.80 (0.73) | 1.56 (0.67) | <0.05 |
| Frequency | 3.08 (0.81) | 2.16 (0.87) | <0.05 |
| Urgency | 2.85 (0.99) | 1.64 (0.45) | <0.05 |
| Nocturia | 1.06 (0.54) | 1.01 (0.23) | 0.87 |
| Quality of life | 2.81 (0.77) | 1.44 (0.54) | <0.05 |
| Diclofenac use in 1 week, median (range) | 3 (1-6) | 3 (1-5) | 1.12 |
Data is presented as mean (SD); SD: Standard deviation; VAS: Visual analogue scale
Discussion
The present study assessed the effect of deflazacort in combination with silodosin, on stent related symptoms following ureteroscopy. Most common side effect associated with ureteral stent placement is pain and multiple factors have been linked to this stent pain pathophysiology. High pressure transmitted to the renal pelvis during micturition, trigonal irritation caused by the intra-vesical portion of the stent, or lower ureteral spasm are some of these pain contributing factors in patients with ureteral stent placement [12,13]. Based on above theories, medical treatment with alpha blocker alone or with anti-muscarinics have been used to reduce stent related symptoms. Mechanisms of these drugs in relieving ureteral stent related symptoms are through smooth muscle relaxation of lower ureter and trigone, reduction in ureter motility, voiding pressure as well urinary reflux [14,15].
Though incidence of ureteral, perineal and loin pain was lower in patients on silodosin than in those on placebo, there are studies where alpha blockers have been combined with Non-Steroidal Antiinflammatory Drugs (NSAIDS) and anti-cholinergics for better result [16-20]. All these studies could not provide confirmatory complete pain relief and there was a need of proper interventions which will give better relief of symptoms to the patients suffering from pain caused by primary pathology or associated intervention such as ureteroscopy.
At present, there are various treatment options available for relieving stent related symptoms; however, studies demonstrating a definitive treatment or recommendations are lacking. A previous study involving an intravesical instillation of ketorolac, alkalinised lidocaine, or oxybutynin in bladder was associated with significant improvement in early pain scores [21]. The role of NSAIDS was probably due to its inhibitory action on ureteral contractility, but its anti-inflammatory role was not much pronounced in this setting. Another study done by Krambeck AE et al., indicated role of ketorolac loaded ureteric stent in reducing pain as compared to controls [22]. A recent study demonstrated combination therapy with tamsulosin and oxybutynin improved irritative symptoms, work performance and sexual matters and recommended combination therapy should be considered for patients who complained of stent related symptoms [23].
The present study hypothesised that steroid being an anti-inflammatory drug, could have a role in alleviating ureteric colic like symptoms in patients with DJ stent. The use of steroids can reduce the risk of edema through inhibition of the inflammation of mucosa caused due to presence of stent in ureter. Movement of stent during patient position aggravates this edematous reaction. Friction between stent and edematous mucosa leads to activation of nociceptors leading to renal colic type of pain. With respect to urological application, deflazacort has been used along with alpha blocker for medical stone expulsive therapy [9]. Deflazacort is a glucocorticoid and it has faster and potent anti-inflammatory action that can be achieved at a low dose [24].
In the present study, patients receiving deflazacort as an adjunct to silodosin reported a significantly better QoL and pain relief, significant reduction in frequency and urgency scores; while similar nocturia score as compared to patients receiving silodosin only. On comparison of VAS pain and voiding flank pain scores between the two groups, it was evident that silodosin in combination with corticosteroid (deflazacort) had better outcomes in comparison to silodosin only group (p<0.05). These observations suggest pain relief with addition of deflazacort to silodosin. This pain relief can be attributed to the anti-inflammatory action of deflazacort on ureteral mucosa. However, due to side effects of long-term steroid therapy, it was given for two weeks period and patient compliance was assured in all cases.
Although there are no direct studies which assessed efficacy of silodosin plus deflazacort in relieving stent related symptoms, the observations of this study are in concordance with the previous studies. Dellabella M et al., evaluated the efficacy of tamsulosin with deflazacort in patients with distal stones and reported that the patients receiving this combination therapy had a significantly higher stone-expulsion rate, a shorter stone expulsion time, no drug-related side effects and shorter hospital stays [25]. Another study by Dellabella M et al., also demonstrated additional benefit of steroids as an increase in expulsion rate from 90% with tamsulosin alone to 96.7% in patients receiving the alpha blocker plus deflazacort. The patients taking the tamsulosin/deflazacort combination also decreased their time of stone passage to 72 hours compared with 120 hours in those on tamsulosin monotherapy [10].
Limitation(s)
The current study was limited to a relatively small sample size, although it was powered to assess a moderate effect size, based on similar studies. A non-validated symptom questionnaire and a VAS have been used to measure stent related symptoms, for ease and efficiency of use, as opposed to the 38-item Ureteral Stent Symptom Questionnaire (USSQ).
Conclusion(s)
Overall observations of this study suggest that the combination of silodosin and deflazacort provided safe and effective relief of pain component in stent related LUTS as compared to silodosin alone and can be considered a better option for relief of pain in patients with post ureteroscopy DJ stenting.
Data is presented as mean (SD), unless otherwise specified; PCNL: Percutaneous nephrolithotomy; SD: Standard deviation; URSL: Ureteroscopic lithotripsy
Data is presented as mean (SD); SD: Standard deviation; VAS: Visual analogue scale