Waldenström’s Macroglobulinemia (WM) is a chronic B-cell lymphoproliferative disorder with bone marrow infiltration with small lymphocytes, lymphoplasmacytoid cells and plasma cells and presence of IgM paraprotein in the serum. We report here a case of a 63 years old male patient, who presented with chronic back pain. The pain was initially thought to be due to disc prolapsed, but on further examination of successive bone-marrow aspirations and biopsies and serum electrophoretogram, proved to be WM. The B cell lymphoproliferative disorders are indolent and include Splenic Lymphoma with Villous Lymphocytes (SLVL), Chronic Lymphocytic Leukaemia (CLL) with IgM paraprotein, and Splenic Marginal Zone Lymphoma (SMZL) and Waldenstrom’s macroglobulinaemia. They can be differentiated on the basis of levels of paraprotein, lymphocytic morphology, and degree of marrow involvement in relation to spleen size, as the malignant cells have the same phenotype in this group of diseases which has been highlighted in the index case.
Case Report
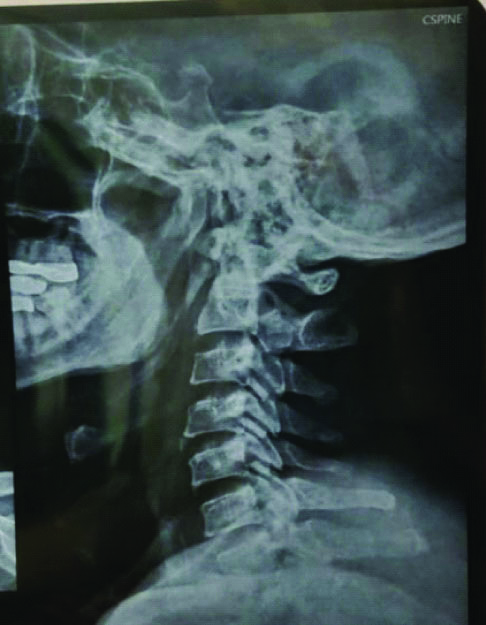
A 63-year-old male reported to the orthopaedics department with the chief complaints of headache, dizziness and back-pain of one week duration. Clinical examination was unremarkable. The family history and past medical history was unremarkable. A CT scan and MRI scan of the lumbar region showed a prolapsed disc and nerve conduction study showed a sensory and motor neuropathy. X-ray chest showed clear lung fields and X-ray cervico-dorsal spine showed cervical spondylosis [Table/Fig-1]. Traction was applied for four sittings and the patient’s neck pain and dizziness resolved to some extent.
X-ray lateral spine showing cervical spondylosis.
Peripheral counts showed haemoglobin of 9.3 gm/dL and normal total and differential count and normal platelet count. The HbA1C, creatinine, thyroid function, lipid profile, serum calcium was normal.
The back-pain relapsed a month after four sittings of traction and peripheral blood counts showed 10% atypical lymphocytes. In view of the atypical cells in the peripheral smear, a bone-marrow aspirate was done to rule out leukaemia which showed 7% blasts, 27% atypical lymphocytes, with scant cytoplasm and nuclei with 1-2 nucleoli (60% lymphocytes, 6% myeloid precursors). Erythropoiesis was micro-normoblastic and myelopoiesis was decreased with normal maturation. Megakaryocytes were decreased in number. An impression of lymphoproliferative disorder was given with a differential diagnosis of Acute Lymphoblastic Leukaemia or Lymphoma in leukaemic phase. The trephine biopsy showed 90% cellularity with Grade III fibrosis and lymphocytosis. The clinician requested further typing of the lymphocytes.
The bone marrow was repeated a month later since no diagnosis was arrived at and the peripheral smear showed atypical lymphocytes which showed a mild lymphocytosis (37%).
At that time, routine biochemical investigations showed a reversal of the Albumin/Globulin ratio (0.9). The urine albumin was 2+. The haemoglobin was 9.3 gm/dL (normal-14-16 gm/dL) and patient had thrombocytopenia (normal-1.5 lac-4.5 lac/cu mm). A MRI showed hepato-splenomegaly with cholelithiasis. Enlarged nodes were seen at the porta-hepatis and peri-pancreatic region with few mediastinal lymph nodes. A diagnosis of tuberculosis; Lymphoma was given. The patient was then referred to a higher centre.
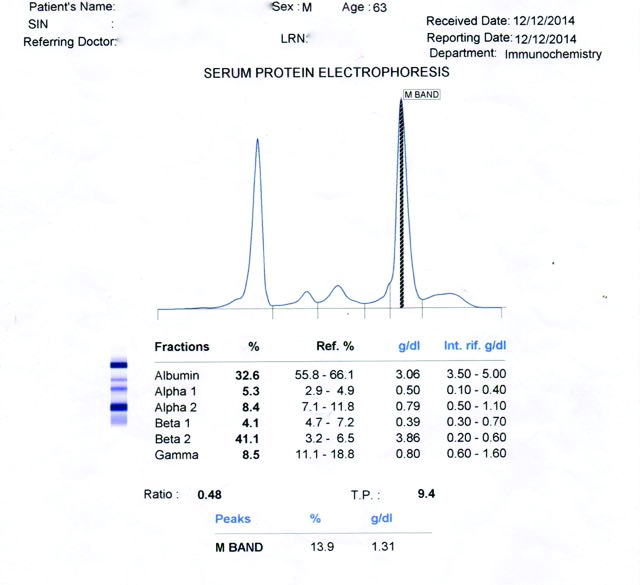
In view of the reversal of A/G ratio (0.75) serum protein electrophoresis was done at the higher centre which showed hyperproteinaemia-total protein 9.8gm/dL and a M band of 1.2 gm/dL, which increased to 15.6gm/dL during the course of next six months. There was hypoalbuminaemia, increase in α1 globulin and β2 globulin. A sharp M band in the β2 globulin was seen. Immunofixation showed an Ig M band [Table/Fig-2].
Serum electrophoretogram.
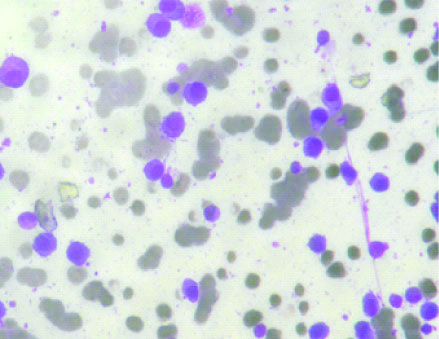
A repeat bone marrow examination was done and lymphoplasmacytic cells and plasma cells were seen [Table/Fig-3]. The immunohisto-chemistry showed positivity for CD20, focal positivity for CD79a, few cells were positive for CD5,CD3,CD43,CD10,CD23 and Cyclin D1 was negative. A few plasma cells were positive for CD138. The Ki67 index was 5-10%. A probable diagnosis of Lympho-plasmacytic Lymphoma/Waldenstrom’s Macroglobulinaemia was rendered. In view of the lymphoplasmacytic infiltrate and M band, the patient was treated with rituximab and cyclophosphamide. The M band fell from 15.6 to 7.3 gm/dL within 6 months. The total protein fell to 8.0 gm/dL.
Bone marrow aspiration smear (Leishman stain 10XX100x) showing lympho-plasmacytic infiltrate.
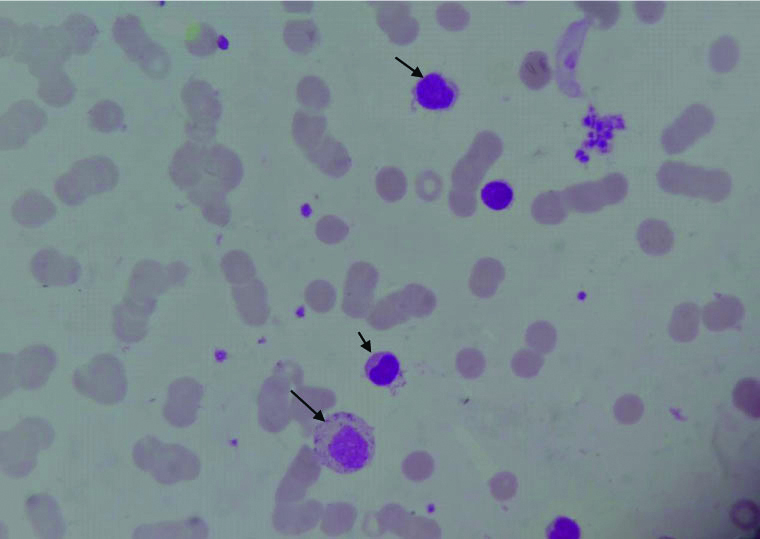
On review of the last peripheral smear done at the time of the last bone-marrow examination, villous lymphocytes were seen [Table/Fig-4]. The final diagnosis was Waldenstrom’s Macroglobulinaemia and the patient was put on rituximab and was doing well till the last follow-up.
Peripheral smear (leishman stain 10XX100x) showing villous lymphocytes.
Discussion
The indolent lymphoproliferative disorders include Waldenstrom’s Macroglobulinemia (WM), CLL with IgM paraprotein, SMZL and SLVL. The differing levels of paraprotein, morphology of lymphocytes, and the degree of marrow involvement and size of the spleen help to differentiate WM from SLVL and SMZL, as the malignant cells have the same phenotype in indolent lymphoproliferative disorders, as is highlighted in the present case [1]. The aetiology is not known
WM is an indolent B-cell lymphoproliferative disorder where small lymphocytes, lymphoplasmacytic cells and plasma cells are seen with serum IgM paraproteinaemia. Neurological manifestations are seen in 10% patients due to infiltration of the peripheral nerves with paraprotein. The present case also had sensory and motor neuropathy [1].
WM presents in the older male, with most patients presenting in the 6th decade. This patient was also in the 6th decade. Peripheral cytopenias due to tumour cell infiltration of bone marrow lead to an increased propensity for infections and bleeding. Other manifestations include splenomegaly, hepatomegaly and lymphadenopathy. Hyperviscosity is seen in 20% of patients and was seen in this case, where the patient had headache and dizziness [1].
In another related entity of Peripheral Splenic Marginal Zone Lymphoma (PSMZL) patients with Lymphoplasmacytic Lymphoma (LPL), have more extensive disease which involves the bone marrow or lymph nodes, spleen, and peripheral blood. They have mild splenomegaly and higher degrees of IgM paraproteinemia with hyperviscosity as was seen in this case [2-8].
The clinical features of splenic MZL overlaps with other indolent lymphomas as in the same manner as this. Splenomegaly is the main feature, which maybe mild in the initial stages. It may be so mild that it is detected by Computed Tomography (CT) scanning as was seen in the present case. Massive splenomegaly is associated with peripheral cytopenias. The splenic hilar lymph nodes are frequently enlarged as was seen in the present case where enlarged nodes were seen at the porta-hepatis and peri-pancreatic region. Peripheral lymphadenopathy is unusual and is seen in disseminated nodal and splenic subtype [6,9,10].
In 15% of the cases of SLVL, villous lymphocytes are seen in the peripheral blood. It is postulated that SLVL is the peripheral spill-over of all splenic MZLs, seen in 75% of cases of SMZL [2,3,9] and between 29% and 75% of the cases of SMZL in another series [6]. It is postulated by some others that it is a sub-group of splenic MZL [6]. SLVL, SMZL and WM are related B-lymphoproliferative disorders and have similar phenotypic cells as was seen in this case.
Bone Marrow (BM) involvement is localised, patchy or diffuse, interstitial or nodular, and sometimes massive in PSMZL and the related LPL. The progression may be slow as was seen in the present case where three bone marrow examination over the course of 2 years established the diagnosis of WM [7,8]. Presently, the bone marrow involvement was interstitial. Bone marrow infiltration was seen in 83-100% of cases of SMZL in one series (mixed pattern) while in another series it was 15% (range, <5%-60%; mean, 25%) [6,9,10].
Raya JM et al., reported similarities between WM and MZL. MZL with plasmacytic differentiation may overlap with LPL and may not constitute a distinct entity. In their study of 16 cases of SMZL, only one patient showed a serum M component, which was of the IgA type [4]. Earlier, reports of patients with PSMZL, having LPL-with monotypic plasma cell component were seen in the spleen. There was an associated serum M component and was associated with auto-immune disorders. A predominant plasma cell infiltration was seen in one patient, after a protracted course. Two consecutive bone marrow biopsies showed plasma cell infiltrate. The disease in this patient could be plasma cell myeloma/WM, thus highlighting the close relationship of WM and PSMZL. The authors have noted a similar relationship in a patient with PNMZL [7,8]. Our patient also had a protracted course before the diagnosis was clinched.
SMZL patients (30%) had a serum M band of IgM. The median monoclonal M band was 2 gm/dL in WM and 0.95 gm/dL in SMZL (p-value <0.001) [10]. Free light chain assay increased free kappa (291 mg/dL and a raised kappa/lambda ratio of 15.9. Serum protein electrophoresis showed a M spike in the gamma region (0.89 gm/dL) [10]. The IgM serum levels are usually lower (<3 gm/dL) in SMZL than in LPL (>3 gm/dL) [3].
The differentiation of nodal MZL and lymphoplasmacytic lymphoma/Waldenström macroglobulinemia can be made by immunophenotyping though can be arbitary as immunophenotyping is similar. Criteria in the WHO classification for nodal MZL and LPL/WM are not distinctive [9]. Most cases show CD10 positive and CD5 negative cells. In this case the CD5 was focally positive and weak and CD23 was negative. In a study of 30 cases of MZL involving the bone-marrow with no serum paraprotein, tissue biopsy/splenectomy aided in classifying the disease entities as nodal MZL/extranodal (MALT lymphoma), or splenic lymphoma [9]. SMZL shows no specific immunophenotype. There is positivity for CD19, CD20, CD22, CD79a, CD79b, FMC7 and IgM. Cells are negative for CD5, CD10, CD43, BCL6, cyclin D1, or CD103 [2]. The immunohistochemistry in our case showed positivity for CD20, focal positivity for CD79a, few cells were positive for CD5. CD3, CD43, CD10, CD23 and Cyclin D1 was negative. A few plasma cells were positive for CD138. Ki67 index was 5-10 %. Thus, the immunophenotyping was overlapping with WM/LPL and SMZL.
Lymphoplasmacytic Lymphoma (LPL)/Waldenstrom’s Macro-globulinaemia is negative for CD5, CD10, and CD23 as was seen in our case [5]. WM cells are IgM-positive, whereas SMZL cells are positive for IgM and IgD [3]. Thus, immunophenotyping and immunohistochemistry also show that there is great overlap between WM/SMZL/LPL.
There have been no reports of overlap of WM and other indolent B cell proliferative disorders in as far as villous lymphocytes being seen in the peripheral blood. This case highlights the overlap in clinical, peripheral blood findings, BM findings and immunohistochemistry.
Waldenstrom’s macroglobulinaemia has an indolent course and responds well to Rituximab.
Conclusion(s)
There is a great overlap between WM/SMZL/LPL in peripheral smear, bone marrow findings, paraproteinaemia, immunophenotyping and immunohostochemical findings as has been highlighted here, where villous lymphocytes a feature of SMZL was seen in a case of WM.
[1]. FE Davies, KC Anderson, Abnormalities in immunoglobulin synthesizing cells. In Blood and Bone Marrow Pathology (Second Edition), Lymphoproliferative disorders associated with an IgM paraprotein. CHAPTER 30Abnormalities in immunoglobulin synthesizing cells 2011 Elsevier Ltd:462-67.10.1016/B978-0-7020-3147-2.00030-421621944 [Google Scholar] [CrossRef] [PubMed]
[2]. Santos TSD, Tavares RS, Farias de DLC, Splenic marginal zone lymphoma: A literature review of diagnostic and therapeutic challengesRev Bras Hematol Hemoter 2017 39(2):146-54.10.1016/j.bjhh.2016.09.01428577652 [Google Scholar] [CrossRef] [PubMed]
[3]. Naeim F, P Nagesh Rao, Wayne W. Grody, Splenic Lymphoma with Villous Lymphocytes. Chapter 15. Mature B-Cell Neoplasms, Morphology, Immunophenotype, Cytogenetics and Molecular Approaches in Hematopathology, 2008Science Direct:297-372.10.1016/B978-0-12-370607-2.00015-6 [Google Scholar] [CrossRef]
[4]. Raya JM, Ruano JA, Bosch JM, Golvano E, Molero T, Lemes A, Splenic marginal zone lymphoma- A clinicopathological study in a series of 16 patientsHematology 2008 13(5):276-81.10.1179/102453308X31606818854089 [Google Scholar] [CrossRef] [PubMed]
[5]. Sohani AR, Zukerberg LR, Small B-cell lymphomas of the spleen: How to tell them apartJournal of Hematopathology 2014 7(3):109-21.10.1007/s12308-014-0208-1 [Google Scholar] [CrossRef]
[6]. Thieblemont C, Non-MALT marginal zone lymphomaAnn Oncol 2008 19(Supplement 4):iv70-iv73.10.1093/annonc/mdn20218519410 [Google Scholar] [CrossRef] [PubMed]
[7]. Audouin J, Le Tourneau A, Molina T, Camilleri-Broët S, Adida C, Comperat E, Patterns of bone marrow involvement in 58 patients presenting primary splenic marginal zone lymphoma with or without circulating villous lymphocytesBr J Haematol 2003 122(3):404-12.10.1046/j.1365-2141.2003.04449.x12877667 [Google Scholar] [CrossRef] [PubMed]
[8]. Jay S, Mra A, Mohamed MM, Jason SC, Splenic marginal zone lymphoma with autoimmunehemolytic anemiaJ Med Therap 2018 2(1):01-05.10.15761/JMT.1000123 [Google Scholar] [CrossRef]
[9]. Inamdar KV, Jeffrey ML, Jorgensen JL, Amin HM, Schlette EJ, Bone marrow involvement by marginal zone B-Cell lymphomas of different typesAm J Clin Pathol 2008 129(5):714-22.10.1309/HRHQFBFTR8B4LXT418426730 [Google Scholar] [CrossRef] [PubMed]
[10]. Arcaini L, Varettoni M, Boveri E, Orlandi E, Rattotti S, Zibellini S, Distinctive clinical and histological features of Waldenström’s macroglobulinemia and splenic marginal zone lymphomaClin Lymphoma, Myeloma Leuk 2011 11(1):103-05.10.3816/CLML.2011.n.02021454204 [Google Scholar] [CrossRef] [PubMed]