The heart as a member of cardiovascular system provides a continuous systematic and pulmonary circulation of blood flow [1]. Coronary artery disease leads to reduction of blood flow to the heart [2]. Chronic hyperglycaemia causes degeneration of small veins affecting optimised function of various organs like kidneys and eyes [3]. Diabetes is directly associated with an increased risk of cardiovascular disease [4]. Angiogenesis is defined as development of new blood vessels from the pre-existing ones. This process is crucial for growth and development of tissues, while it can be affected by diabetes [5,6]. Diabetes disrupts the balance which exists in the activity of inhibitory and stimulatory factors of angiogenesis, especially in cardiac tissue [7]. In fact, diabetes by alteration of VEGF gene expression can reduce the rate of angiogenesis [8]. About 80% of people in developing countries prefer medicinal plants for treatment of disease [9].
Materials and Methods
This experimental study was performed in Department of Anatomical Sciences of Kermanshah University of Medical Sciences from April 2019 to November of 2019. All animals were treated in accordance with guidelines of National Institute of Health for the Care and Use of Laboratory Animals approved by Research Deputy at Kermanshah University of Medical Sciences (Department of Anatomical Sciences, Medical School, Iran) based on WMA Declaration Ethic of Helsinki (Ethic number; IR.KUMS.REC.1397.564).
Sixty-four male Wistar rats (200-250 gm) were prepared from the Pasteur Institute (Tehran, Iran) and housed in animal home under normal conditions (22±2°C, 12 hours light/dark photocycle). They had free access to the water and standard food. All 64 animals were divided into 8 groups (8 animals in each) by random; control group, extract groups (4.5, 9 and 18 mg/kg), diabetic group, and diabetic + extract groups (4.5, 9 and 18 mg/kg). The extract was administered for 60 consecutive days by i.p injection.
Chemicals and Reagents
T. vulgaris was purchased from the centre of medicinal plants (Natural pharmacy health school traditional medicine, Faculty of Traditional Medicine, University of Tehran, Iran). Ethanol, formalin, paraffin and xylol were purchased from the Merck Co. (Germany). STZ was prepared from Sigma Co. (USA). Sodium chloride 0.9% was obtained from the Darou Pakhsh Co. (Tehran, Iran). H2O2 (5%) solution was provided from the Behsaman Co. (Iran). CD31 primary antibody, EnVision secondary antibody, PBS solution, R.S solution, DAB solution, and haematoxylin solution were purchased from the Dako Co. (Denmark). CK-E30634 ELISA kit was purchased from the Biopharm Co. (USA).
Induction of Diabetes Type I
It was achieved by a single i.p dose of STZ (60 mg/kg). This destructive chemical, causes partial or total degradation of pancreatic β cells. Two days later the animals were considered diabetic if the blood glucose was greater than 250 mg/dL. This procedure was repeated twice to confirm the diabetes. The blood sugar was also measured weekly by the end of experiment [15].
Preparation of Hydroalcoholic T. vulgaris Extract
This plant was purchased from a local store (Kermanshah, Iran, spring 2019) and confirmed by a botanist. The plant was cleaned and leaves and stems were dried in the shade and then grounded to produce the PC powder. It was dissolved in 98% ethanol solution, and it was extracted using distilled water and ethanol (1:1 V/V) as a solvent. The solution was filtered and concentrated under the reduced pressure on a rotary evaporator. It was finally frozen at -80°C for future application. If needed, it was dissolved in water and prepared freshly for daily experimental use. The hydroalcoholic extract of T. vulgaris was administrated (doses of 4.5, 9, and 18 mg/kg) for one month by i.p injection [11,12].
Measurement of VEGF: VEGF serum concentration was measured by ELISA technique and CK-E30634 kit (Biopharm, USA). Samples and standards were prepared. A 40 μL of rat standard serum sample was added, followed by 10 μL of biotin solution and 50 μL of HRP linked polyclonal antibody. They were added to each well of microplate coated with monoclonal antibodies and incubated at 37°C for 60 minutes. They were washed 5 times with washing solution, and the solution of chromogen A and B were added to the wells and incubated at 37°C for 10 minutes. A 50 μL of Stop solution was added to the wells for colour resuscitation. Finally, the optimum density of VEGF concentration was determined (ng/L) at the 450 nm wavelength using a Microplate Reader [16].
Heart sampling and blood serum preparation: Under anaesthesia, the thoracotomy procedure was done and the blood samples were gathered directly from the heart for measurement of serum VEGF. The heart was removed, washed in normal saline solution and fixed in formalin. The cardiac tissues underwent processing by an autotechnicon machine, and they were embedded in paraffin to assess the capillary density using immunohistochemistry technique [16].
Griess technique: Nitrite oxide was measured by the Griess assay using the microplate technique. The mixture of zinc sulfate powder (6 mg) and the serum samples (400 μL) was vortexed for one minute and then centrifuged (4°C, 10 minutes, 12,000 rpm). The supernatant was used for nitrite oxide measurement. A 50 μL of sample was added to 100 μL of Griess reagent (Sigma; USA) and incubated (30 minutes at room temperature). As per manufacturer protocol by an ELISA reader (Hyperion; USA), Optical Density (OD) of the sample at wavelength of 540 nm was measured [17].
Measurement of capillary density: The primary antibody CD31 was used as an indicator of endothelial cells in cardiac capillaries. Cardiac paraffin blocks were cut into 3 μm sections. The slides were passed through incubator and various solutions respectively as follows; incubated in oven and then in Xylol, placed in PBS and R.S solutions, placed in distilled water, H2O2 solution (5%), and finally washed in distilled water and PBS solutions. An appropriate amount of antibody was placed on the slices while adding the primary antibody. Then the slides were washed. The secondary antibody was also added to the slices, and they were washed and placed in a PBS buffer. In the next step, the chromogen was placed on the slices and washing process was applied with distilled water and PBS solutions. Haematoxylin was added to the slices. Finally, the slides were dehydrated and placed in xylol. Ten microscopic fields from each tissue were selected, and the capillary density was expressed as the number of endothelial cells per mm2 [16].
Statistical Analysis
The Kruskal-Wallis test was used for examination of data normality. Data were analysed using SPSS software (version 16.0). The Unpaired t-test was used, and one-way analysis of variance (one-way ANOVA) was followed by Tukey’s Post-Hoc tests. The p-value <0.05 (p<0.05) was considered as statistically significant. All data were expressed as mean±SEM.
Results
At the beginning of this study, there were 64 animals (8 groups), 48 (6 groups) of which were treated with the extract. Animals of diabetic group which died following diabetes induction were replaced again with new animals.
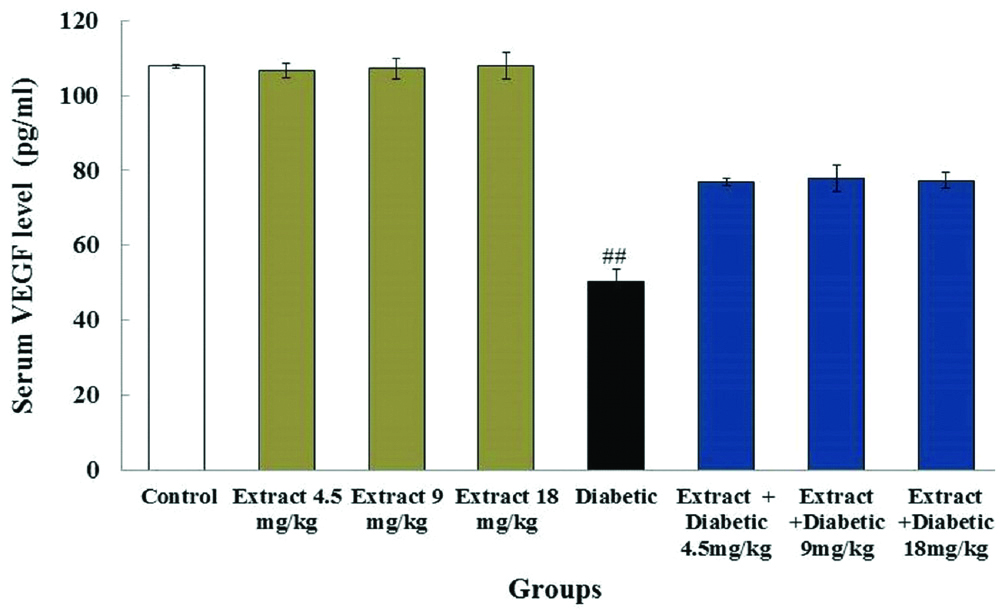
Serum level of VEGF: The level of serum VEGF in diabetic group (50.5±3.1 pg/mL) reduced significantly compared to the control group (107.87±0.6 pg/mL) (p<0.05). Extract administration with doses of 4.5, 9 and 18 mg/kg showed no significant effects on VEGF expression in the extract groups (106.62±2.4 pg/mL, 107.25±2.2 pg/mL and 108.07±3.5 pg/mL) in comparison to the control group (107.87±0.6 pg/mL). Also, no significant alteration in VEGF serum concentration were detected among the diabetic group (50.5±3.1 pg/mL) and the diabetic + extract groups (76.8±1.4 pg/mL, 77.87±3.6 pg/mL and 77.33±2.3 pg/mL) (p>0.05) [Table/Fig-1].
The mean concentrations of serum VEGF in experimental groups.
##Significant difference in the diabetic group compared to the control group (p<0.05)
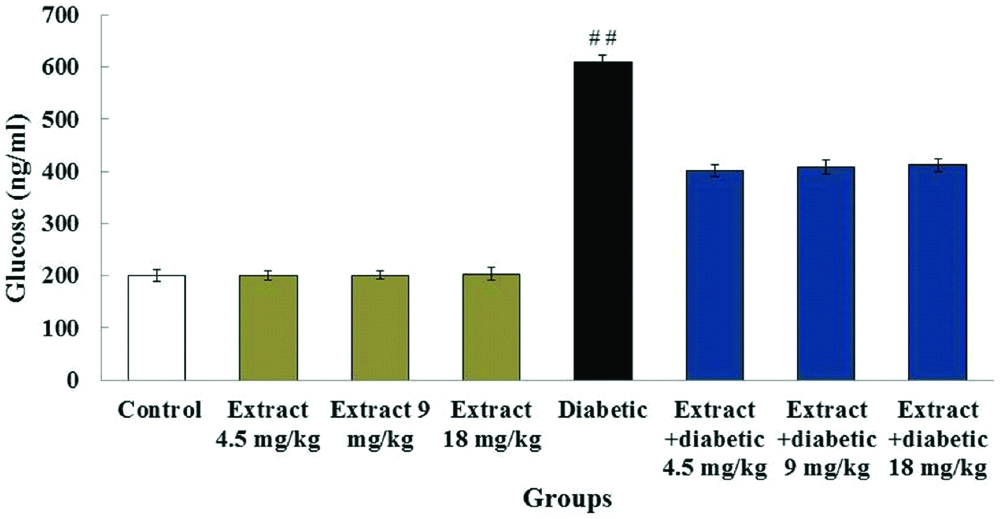
Levels of blood glucose: The comparison of mean blood glucose in different experimental groups were shown at various stages; the beginning of the experiment and first to fourth weeks of treatment. The blood glucose levels in diabetic (609.5±13.1 ng/mL) and diabetic + extract groups (402.5±11.4 ng/mL, 408.12±14.2 ng/mL and 411.87±11.5 ng/mL) were significantly higher than the control (200.37±12.4 ng/mL) and extract groups (200.75±9.5 ng/mL, 198.37±8.6 ng/mL and 203.23±12.7 ng/mL) (p<0.05). However, there were no significant differences in blood glucose levels in control group (99.37±12.4 ng/mL) in comparison with the extract groups (99.75±9.5 ng/mL, 99.37±8.6 ng/mL and 99.23±12.7 ng/mL) as well as the diabetic group (509.5±13.1 ng/mL) compared to the diabetic + extract groups (492.5±11.4 ng/mL, 482.12±14.2 ng/mL and 480.87±11.5 ng/mL) (p>0.05) [Table/Fig-2].
Effects of diabetes and T. vulgaris on the mean blood glucose levels in rats (n=8 for each group).
##Significant difference in the diabetic group compared to the control group (p<0.05)
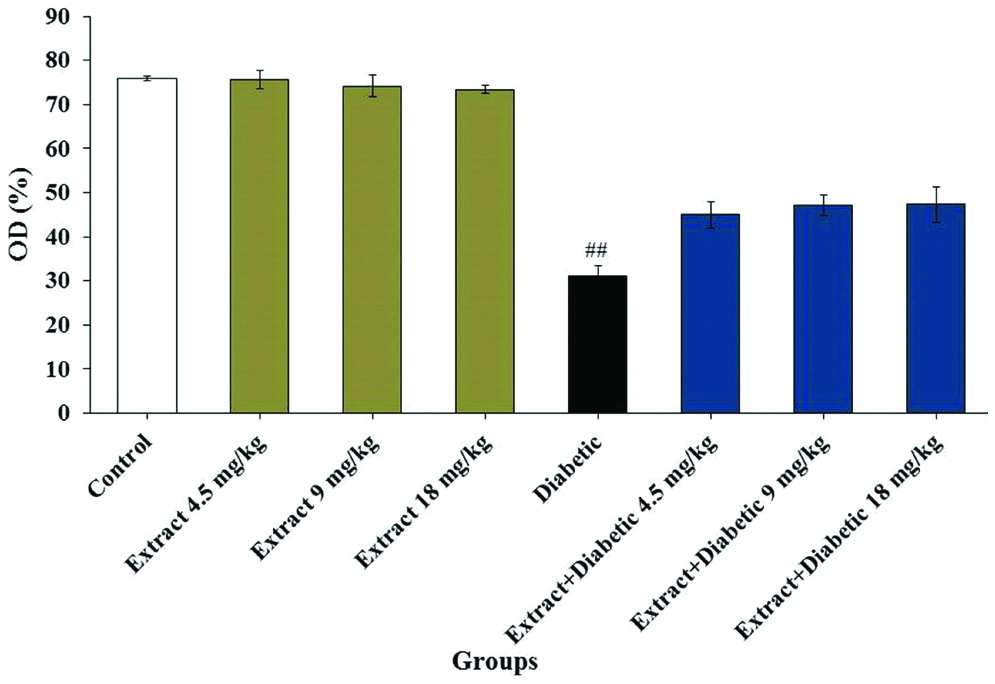
Nitrite oxide: Biochemical analysis of serum nitrite oxide showed a significant decrease in diabetic group (31.13±2.4) compared to the control group (75.94±0.6) (p<0.05). The mean nitrite oxide level was not significant in all extract groups (75.63±2.1, 74.19±2.4 and 73.4±0.8) compared to the control group (75.94±0.6) (p>0.05). Also, this value was not significant in diabetic + extract groups (45.03±3.2, 47.18±2.4 and 47.38±4.1) in all doses compared to the diabetic group (31.13±2.4) (p>0.05) [Table/Fig-3].
Effects of T. vulgaris and diabetes on the mean nitrite oxide levels.
##Significant difference compared to the control group (p<0.05). OD: Optic Density
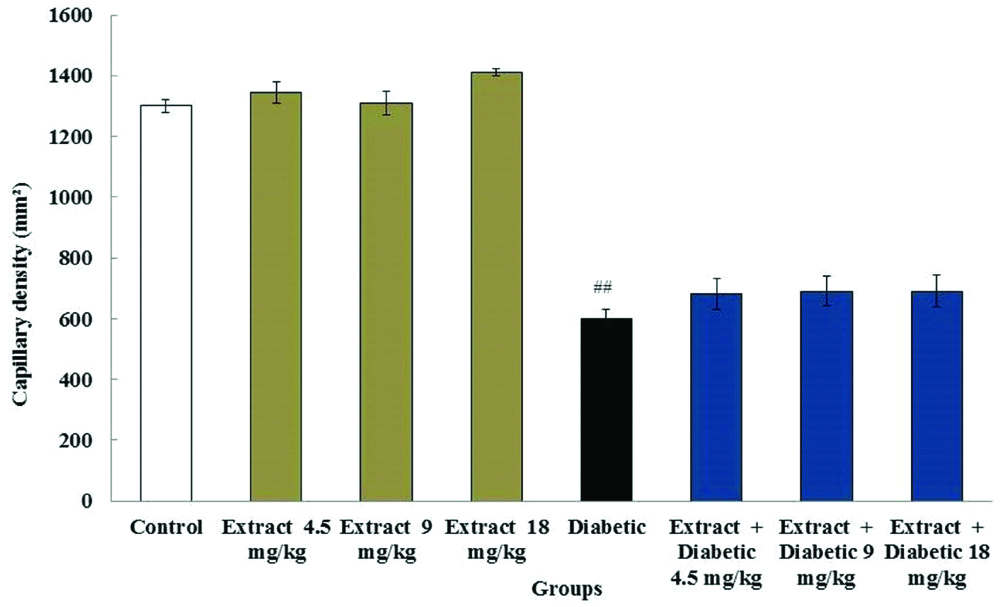
Capillary density in heart: Results showed a significant alteration of capillary density in diabetic group (1050±30 mm3) compared to the control group (2100±22 mm3) (p<0.05). Findings showed no significant differences in the field of capillary density between the extract groups (2110±40 mm3, 2111±11 mm3 and 2104±34 mm3) and control group (2100±22 mm3) as well as the diabetic + extract groups (1480±51 mm3, 1490±48 mm3 and 1491±52 mm3) compared to diabetic rats (p>0.05) [Table/Fig-4].
Effect of diabetes and T. vulgaris on the myocardial capillary density.
##Significant difference in diabetic group compared to the control group (p<0.05)
Discussion
As the obtained results in this study showed that diabetes reduces the cardiac capillary density, serum levels of VEGF and nitrite oxide compared to the control group. T. vulgaris at various concentrations of 4.5, 9, and 18 mg/kg increased the cardiac capillary density in diabetic groups receiving extract, but it was not statistically significant. Also, the capillary density rate in the extract groups was not significantly different compared to the control group. The findings of the present research showed no significant alteration in serum VEGF of diabetic group compared to the control group, which is consistent with some other studies [18-20].
According to the study of Hazarika S et al., the VEGF signalling in diabetic animals was lower expressed than the control group in the absence of ischaemia, which is in line with the results of the present study [19]. The results of Gao L and Yu DM, were in agreement with the findings of the present research indicating that the capillary density and revascularisation in the limbs of diabetic rats were decreased, with reduced mRNA and protein expression of eNOS, VEGF, and bFGF [20]. T. vulgaris has various biochemical compounds like terpenes, glycosides, and flavonoids (including kaempferol and quercetin) [21,22]. Flavonoids inhibit angiogenesis process by reduction in VEGF expression [23,24]. In a study, the quercetin presented anti-angiogenesis effects with less proliferation and migration of vascular endothelial cells by reduced expression and activity of 2-metalloproteinase of matrix [25]. The results of this study also detected that the i.p prescription of T. vulgaris hydroalcoholic extract increases cardiac capillary density in diabetic rats, while it slightly reduces VEGF in experimental groups compared to the control group, but it is not statistically significant. VEGF is an effective inducer of angiogenesis in various models [26]. The findings of Li Y et al., confirmed the results of the present study indicating that the diabetes could decrease the plasma VEGF level [27]. As it was stated by previous studies, this consequence returns to VEGF tolerance of diabetes-induced vascular complications [16]. The probable reason for this phenomenon is the type of diabetes (Type I) induced for animal studies, which caused more severe complications and disabilities and less tolerance against diabetes. Thus, the herb extract exerts no therapeutic effects for alleviation of diabetes complications.
Also, this study revealed that the extract of T. vulgaris did not increase blood glucose levels significantly in extract groups and also had no decreasing effects on blood glucose levels in diabetic rats. However, its administration in diabetic+extract groups with specific doses reduced the blood glucose level at the end of the experiment (4th week) but not significantly . Science has revealed that the polysaccharides, flavonoids, polypeptides, and alkaloids existing in medicinal plants can justify the nature of hypoglycaemic and hypolipidemic [28,29]. Also, some studies have reported the positive-effects of medicinal plants with antioxidant properties [30].
The results of a study by Su HC et al., did not confirm the results of the present study indicating that the administration of flavonoids may increase the glucose uptake by hepatocytes, adipocytes and muscle cells [31]. A part of the hypoglycaemic effects of flavonoids administration seems to be related to the increased activity of hepatic hexokinase and glucokinase enzymes, as well as their insulin-like effect which reduces the symptoms of diabetes mellitus [32]. In this study, it was concluded that the serum nitrite oxide level in diabetic rats was lower than the control group. The statistical analysis demonstrated that the capillary density in myocardial tissue was correlated with the high serum angiogenic markers. Angiogenesis is controlled by a number of pro-angiogenic and anti-angiogenic factors released in extracellular matrix. Both nitrite oxide and VEGF have crucial roles in cellular process angiogenesis [33]. Nitrite oxide not only has a direct effect on angiogenesis but also affects other angiogenic growth factors by producing excessive nitrite oxide [34]. In endothelial nitrite oxide synthase-deficient rats, a decrease in nitrite oxide production can disrupt coronary vessels development and angiogenesis process [35]. The results of Silva AM et al., were in parallel with the findings of the present research, indicating that the serum nitrite oxide concentration in diabetic rats was lower [36]. Salahshoor MR et al., reported that the T. vulgaris administrated for male Wistar rats attenuated the nitrite oxide level which did not confirm the results of the current research [11].
The lack of therapeutic effect of T. vulgaris extract on blood glucose reduction may return to the non-toxic i.p doses, the amount of Flavonol active ingredient and the duration of the extract administration. It is also suspected that the active ingredients of T. vulgaris do not enter into the blood circulation due to low dose of active ingredient or not being absorbed through the i.p administration, or they have been metabolised into inactive hepatic metabolites. Another possible reason is the slight reduction in blood glucose that is neutralised by the absorbable carbohydrates which are normally found in the T. vulgaris extract. It is also possible that the blood glucose reducing substances along with their additives maybe existed in T. vulgaris extract to prevent blood glucose levels in the experimental rats.
Limitation(s)
Some mechanisms of action of this extract on coronary angiogenesis process were unclear. Animal death after diabetes induction; the number of animals in each group, ultimately remained unchanged, and in each group, 8 animals were tested. Dead animals following induction of diabetes were replaced again. Nitrite oxide measurement is strongly due to its very high volatility.
Conclusion(s)
T. vulgaris extract at all the dosages did not show significant effects on blood glucose level, nitrite oxide, serum capillary density, and VEGF in control and diabetic groups. This study showed that there are several factors leading to non-therapeutic effects of T. vulgaris to prevent the incidence of cardiovascular disorders in male Wistar rats. These factors are the type of diabetes, the dose of drug, and duration of drug administration. In future studies must be taken for complete association of the molecular interaction between T. vulgaris, diabetes and coronary angiogenesis.