Introduction
The S-ECC is one of the most prevalent disease in children worldwide [1]. In Thailand, the prevalence of S-ECC in rural and capital areas is higher than 70% [2]. ECC causes not only local pain but also affects general growth and development, loss of self-esteem and might lead to psychological problems [1,3].
ECC results from an interaction between the acidogenic bacteria, sucrose, and host susceptibility [3,4]. Social and behaviour habits are contributing risk factors [3,4]. In the oral cavity, there are biofilm (dental plaque) which comprises of more than 800 species of microorganisms living in a complex community. It changes over time and the microorganisms population can shift between healthy and pathological environment when factor such as sugar is enhanced [4].
Streptococcus mutans and Streptococcus sobrinus are causative pathogen of dental caries [3-7]. S.mutans is commonly isolated microorganism from dental plaque [6-10]. Not only it is aciduric and acidogenic but also has the capability to adhere and deposit on the tooth surfaces. In the presence glucosyltransferases (Gtfs) (an enzyme of S.mutans), sucrose molecules are cleaved and the glucose component is polymerised into adherent glucans [10]. S.mutans is also able to generate the acid from carbohydrate and tolerate low pH environment [9-11].
S.sobrinus and S.mutans are different in many biochemical characteristics and virulence factors. S.sobrinus has greater acidogenic capacity than S.mutans [10,11]. The attachment of S.sobrinus happens when the pellicles are exposed to sucrose but this does not occur in S.mutans. The cell-associated Gtfs activity of S.sobrinus formed a higher percentage than that of S.mutans [11].
Previous studies showed that the prevalence of S.mutans in dental plaque of caries-active subjects was higher than S.sobrinus [12-14]. Numerous studies stated that children who were infected with both S.mutans and S.sobrinus have greater incidences of caries than those with S.mutans alone [13-15]. In contrast, study in Thai children found that when subjects were infected by both of them, the caries prevalence was the same as subjects infected by S.sobrinus alone [7].
Previous studies have reported the antagonistic relationship between S. mutans and S. sanguinis. They suggested that S. sanguinis delay the colonisation of S. mutans in the oral cavity and that caries-free children were colonised by high amounts of S. sanguinis. The interaction between S. mutans and S. sanguinis was also associated with caries outcome [16-19]. Previous studies have shown the relationship between S. mutans and S. sobrinus or S.mutans and S.sanguinis in dental plaque and saliva, none of them provided information on both sources in one study especially in Thai children [6,7].
Quantitative real-time PCR provides an accurate result and is a sensitive method for the detection and quantification of bacterial species [15]. This study aimed to detect S.mutans, S.sobrinus and S.sanguinis in dental plaque and saliva samples using real-time PCR from S-ECC and caries-free groups of Thai children, and to analyse the association between these bacteria and other caries-associated factors. The hypothesis is that the quantities of S.mutans, S.sobrinus and S.sanguinis from S-ECC and caries-free groups should be different.
Materials and Methods
This cross-sectional study was conducted between January 2015 to December 2017 at Faculty of Dentistry, Mahidol University, Bangkok, Thailand. The approval from Human Institutional Review Board of the Faculty of Dentistry and the Faculty of Pharmacy, Mahidol University (MU-DT/PYIRB 2009/266.0910, 2013/027.2606) was obtained prior to initiation of the study. Sample size was calculated based on the previous study [6], with α=0.05 and power of 80%, using the software package Primer of Biostatistics (McGraw-Hill, NY, USA).
Subject Selection
Total subjects were 120 (caries-free=60, S-ECC=60) Thai children aged two to five-year-old. All subjects were randomly selected from public schools in Pathumthani province, Thailand. Consent forms were signed. A clinical examination was performed by 2 paediatric dental residents. They were calibrated for clinical examination (kappa co-efficiency=0.85). The diagnosis of S-ECC was based on the AAPD [20]. Children with any systemic disease(s), on any kind of antibiotics, had professional fluoride application or any dental treatment within 3 months prior to the sample collection period were excluded.
Clinical Examination, Plaque Index and Modified Gingival Index
Recorded dmft score and plaque index used a modified debris index of simplified oral hygiene index for deciduous dentition [21-23]. Gingival inflammation was recorded on a 0-4 scale following the modified gingival index [22,23].
The questionnaire: All participants’ parents or caretakers were asked to complete the questionnaire by face-to-face interview. All questions were close ended. Besides the parents’ general information, 3 categories were examined: 1) Child’s general information; 2) Parental attitude towards child’s diet: a) Is your child still bottle feeding?; b) Did your child ever have breast and/or bottle feeding ad lib?; c) Did your child breast and/or bottle feed ad lib and fall asleep?; d) Did you always give your child water after breast or bottle feeding?; e) What type of snacks does your child have per day?; f)Type and frequency of snacks; 3. Parent’s attitude and behaviour in child’s oral hygiene care: a) How many times per day do you brush your child’s teeth?; b) When did you last take your child to the dentist. The Cronbach’s alpha coefficient was 0.7, which is acceptable [6].
Plaque and Saliva Sample Collection
Supra-gingival overnight plaque samples were collected from bucco-gingival surfaces of all teeth using a sterile toothpick and released in 1 mL of TrisBase and EDTA buffer, then they were asked to expectorate saliva into a cup.
DNA Extraction
DNA was extracted based on enzymatic lysis using a commercial kit (Flavogen, Taiwan) as previously described [6]. Extracted DNA concentration and purity were measured by a spectrophotometer at 260 nm/280 nm (Nanodrop 2000C® Thermo Scientific, Delaware, USA).
Culture Condition and Standard Strains
S. mutans ATCC 25175, S. sobrinus ATCC 6715 and S. sanguinis OMZ 2176 strains were cultured in brain heart infusion agar and broth. Genomic DNA was extracted from the overnight culture as described above.
Conventional PCR
All extracted DNA samples were confirmed with 16srRNA universal primers [Table/Fig-1] [24-27]. Each reaction mixture (total volume of 25 μL) contained 2 μL of DNA sample, 16.5 μL of nuclease-free water, 1 μL of 10 mM deoxynucleoside triphosphate (dNTP), 1 μL of each primer, 1.5 μL of 50 mM MgCl2, 2.5 μL of 10X PCR buffer minus Mg, and 0.5 μL of Taq DNA polymerase (KAPA Biosystems, USA) using Thermocycle (GeneAmp PCR System 9600 PCR machine, PerkinElmer, CA, USA) as previously described [28].
Primers used in this study [24-27].
| Primer name | | Nucleotide sequence (5’ to 3’) | Expected amplicon (bp) | Annealing Temp (°c) | Ref |
|---|
| Universal 16SBAC | F | 5-TGG AGC ATG TGG TTT AAT TCG A-3′ | 160 | 56.7 | Sinsimer D et al., [24] |
| R | 5-TGC GGG ACTTAACCC AAC A-3′ |
| S. mutans | F5 | 5’-AGCCATGCGCAATCA ACA GGT T-3’ | 415 | 59 | Yano A et al., [25] |
| R4 | 5’-CGCAACGCGAACATC TTG ATC AG-3’ |
| S. sobrinus | SobF | 5’-CGCACTTGCTCCAGTGTTACTAA-3’ | 546 | 51 | Sato T et al., [26] |
| SobR | 5’-GCC TTT AAC TTC AGA CTT AC-3’ |
| S. sanguinis | MKP-F | 5’-GGATAGTGGCTCAGGGCAGCCAGTT-3 | 313 | 61.5 | Hoshino T et al., [27] |
| MKP-R | 5’-GAACAGTTGCTGGACTTGCTTGTC-3’ |
Quantitative Real-time PCR
Using specific primers [Table/Fig-1], the reaction mixture (total volume of 20 μL) contained 8.2 μL of water, 10 μL of 2X KAPA SYBR® FAST qPCR Master Mix, 0.4 μL of 10 μM forward and reverse primer, and 1 μL of standard bacteria DNA. We set the thermocycler (C1000™ Thermal cycler and CFX 96 Real-time System) for 40 cycles. Each cycle consisted of enzyme activation at 95°C for 3 minutes, denaturing at 95°C for 3 seconds, annealing for 20 seconds and extension for 30 seconds. Melting curves were generated from 60°C to 95°C and read every 0.5°C for 5 seconds [6].
Agarose Gel Electrophoresis
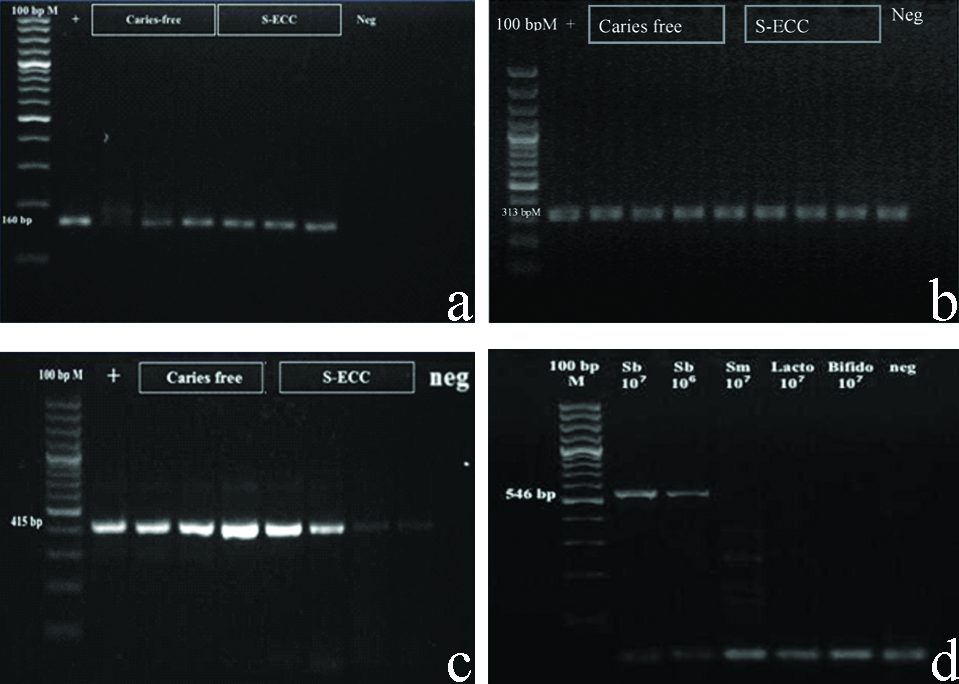
Amplified PCR products were checked with 1.5-2% agarose gel which stained with ethidium bromide and gel image were captured with a digital imaging system [Table/Fig-2] (Molecular Imager ®Gel docTM Systems, Bio-Rad Laboratories Inc., CA, USA) [6].
Agarose gel electrophoresis of real-time PCR products (a) universal primers, (b) specific primers for S. sanguinis and (c) specific primers for S. mutans (d) specific primers for S. sobrinus.
Statistical Analysis
SPSS 16.0 software (Microsoft Corporation, USA) was used to record and analyse the data. Kolmogorov-Smirnov and Shapiro-wink tests (p<0.001) were used to assess data distribution. The different amounts of each bacterium were analysed by Mann-Whitney U test (p<0.05). The correlation between amounts of each bacterium and other clinical factors were analysed by Spearman’s correlation test (p<0.05). The association between caries status and demographic, socioeconomic, diet, and other factors were analysed by Pearson’s Chi-Square test (p<0.05).
Results
Participants
Total subjects were 120 (S-ECC=60, CF=60). Mean age of the children was 3.56±0.53 years. [Table/Fig-3,4 and 5] showed that the guardian’s demographic data, habit of milk bottle and breast feedings, oral hygiene care and consumption of cariogenic snacks were different between two groups.
Demographic characteristics of subjects in both groups.
| Variables | Caries-free | S-ECC | p-value1 |
|---|
| n (%) | n (%) |
|---|
| Children’s gender |
| Male | 28 (46.7) | 28 (46.7) | 1.0 |
| Female | 32 (53.3) | 32 (53.3) |
| Guardian’s education levels |
| Primary school | 4 (6.7) | 16 (26.7) | 0.001* |
| High school or diploma | 23 (38.3) | 35 (58.3) |
| ≥Bachelor degree | 33 (55) | 9 (15) |
| Guardian’s occupation |
| Worker for government or private company | 32 (53.3) | 14 (23.3) | 0.001* |
| Merchant | 17 (28.4) | 13 (21.7) |
| Employee | 5 (8.3) | 23 (38.3) |
| Housekeeper | 6 (10) | 10 (16.7) |
| Relationship with the child |
| Parents | 50 (83.3) | 37 (61.7) | 0.02* |
| Grandparents or senior relative | 10 (16.7) | 21 (35) |
| Babysitters | 0 (0) | 2 (3.3) |
| Monthly family income |
| <10,000 baht | 5 (8.3) | 10 (16.7) | 0.001* |
| 10,001-20,000 baht | 17 (28.3) | 37 (61.6) |
| ≥20,000 baht | 38 (63.4) | 13 (21.7) |
| Family member’s smoking |
| Yes | 11 (18.3) | 38 (63.3) | 0.011* |
| No | 49 (81.7) | 22 (36.7) |
1Pearson’s Chi-square test. *p-value <0.05
Feeding pattern and oral hygiene care in 2 groups.
| Variables | Caries-free | S-ECC | p-value1 |
|---|
| n (%) | n (%) |
|---|
| Milk bottle |
| Yes | 18 (30) | 45 (75) | 0.001* |
| No | 42 (70) | 15 (25) |
| Frequency of milk bottle feeding |
| 1 time/day | 7 (38.9) | 13 (28.9) | 0.555 |
| 2-3 times/day | 8 (44.4) | 19 (42.2) |
| >3 times/day | 3 (16.7) | 13 (28.9) |
| Duration of milk bottle feeding |
| <10 minute/each feeding | 11 (61.1) | 14 (31.1) | 0.043* |
| 10-30 minutes/each feeding | 5 (27.8) | 13 (28.9) |
| >30 minute/each feeding | 2 (11.1) | 18 (40) |
| Breast milk |
| Yes | 4 (6.7) | 18 (30) | 0.001* |
| No | 56 (93.3) | 42 (70) |
| Frequency of breast feeding |
| 1 time/day | 3 (75) | 13 (72.2) | 0.751 |
| 2-3 times/day | 1 (25) | 3 (16.7) |
| >3 times/day | 0 (0) | 2 (11.1) |
| Duration of breast feeding |
| <10 mins/time | 4 (100) | 12 (66.7) | 0.400 |
| 10-30 mins/time | 0 (0) | 5 (27.8) |
| >30 mins/time | 0 (0) | 1 (5.5) |
| Sleep with bottle of milk |
| Yes | 13 (21.7) | 41 (68.3) | 0.001* |
| No | 47 (78.3) | 19 (31.7) |
| Brushing frequency |
| >1 time/day | 53 (88.3) | 33 (55) | 0.001* |
| once a day | 6 (10) | 20 (33.3) |
| once in 2 days | 1 (1.7) | 7 (11.7) |
| Drinking water after milk feeding |
| Always | 39 (65) | 20 (33.3) | 0.002* |
| Sometimes | 18 (30) | 31 (51.7) |
| Never | 3 (5) | 9 (15) |
| Dental treatment |
| Regular | 23 (38.3) | 5 (8.3) | 0.001* |
| Irregular | 6 (10) | 18 (30) |
| Never | 31 (51.7) | 37 (61.7) |
1Pearson’s Chi-square test. *p-value <0.05
Type and frequency of snacks consumption in both groups.
| Variables | Caries-free | S-ECC | p-value1 |
|---|
| n (%) | n (%) |
|---|
| Frequency of protein |
| None | 11 (18.3) | 5 (8.3) | 0.092 |
| Only in meal | 33 (55) | 30 (50) |
| Between meal ≥1 time/day | 16 (26.7) | 25 (41.7) |
| Frequency of fruit |
| None | 7 (11.7) | 13 (21.7) | 0.069 |
| Only in meal | 32 (53.3) | 18 (30) |
| Between meal ≥1 time/day | 21 (35) | 29 (48.3) |
| Frequency of sugar coated snacks |
| None | 13 (21.7) | 6 (10) | 0.001* |
| Only in meal | 37 (61.6) | 21 (35) |
| Between meal ≥1 time/day | 10 (16.7) | 33 (55) |
| Frequency of soft drink in one day |
| None | 27 (45) | 3 (5) | 0.001* |
| Only in meal | 14 (23.3) | 15 (25) |
| Between meal ≥1 time/day | 19 (31.7) | 42 (70) |
| Frequency of hard candies in one day |
| None | 32 (53.3) | 12 (20) | 0.001* |
| Only in meal | 15 (25) | 12 (20) |
| Between meal ≥1 time/day | 13 (21.7) | 36 (60) |
| Frequency of potato chip in one day |
| None | 10 (31.3) | 2 (6.7) | 0.008* |
| Only in meal | 13 (40.6) | 9 (30) |
| Between meal ≥1 time/day | 9 (28.1) | 19 (63.3) |
| Frequency of Thai dessert in one day |
| None | 19 (59.3) | 8 (26.7) | 0.034* |
| Only in meal | 6 (18.8) | 11 (36.7) |
| Between meal ≥1 time/day | 7 (21.9) | 11 (36.6) |
1Pearson’s Chi-square test. *p-value <0.05
Conventional PCR and Quantification Real-Time PCR of S. mutans, S. sobrinus and S. sanguinis
There was a 100% detection rate by the universal primers. [Table/Fig-6] shows the comparison of bacterial levels between the two groups. Both plaque and gingival indices were different between the two groups [Table/Fig-7]. [Table/Fig-8] shows the correlation of clinical parameters and microbial finding between two groups. When compared between plaque and saliva, S. mutans levels were not significantly different.
Comparison of bacterial levels between caries free and S-ECC groups.
| Bacterial | Caries-free | S-ECC | p-value1 |
|---|
| S. mutan |
| Mature plaque | 2.87×104±7.96×104 | 1.90×105±5.75×105 | 0.005* |
| Total bacteria |
| Mature plaque | 5.33×107±4.24×107 | 3.85×107±3.51×107 | 0.017* |
| S. mutans/total bacteria |
| Mature plaque | 6.91×10-4±2.23×10-3 | 5.65×10-3±1.36×10-2 | 0.003* |
| S. sobrinus |
| Mature plaque | 59.34±1.58×102 | 7.45×102±1.59×103 | <0.001* |
| S. sobrinus/total bacteria |
| Mature plaque | 3.9×10-6±1.27×10-5 | 4.67×10-5±1.24×10-4 | <0.001* |
| S. sanguinis |
| Saliva | 1.3×107±5.3×106 | 2.4×106±9.3×105 | 0.004* |
| S. mutans |
| Saliva | 4.8×104±1.1×104 | 5×106±1.5×106 | 0.001* |
| S. sanguinis/S. mutans |
| Saliva | 1.8×103±9.9×102 | 6±3.7 | 0.001* |
| S. sanguinis/total bacteria |
| Saliva | 6×10-3±2×10-3 | 1.1×10-3±3.3×10-4 | 0.001* |
| S. mutans/total bacteria |
| Saliva | 1.1×10-3±1.1×10-3 | 3.2×10-3±1×10-3 | 0.001* |
1Nonparametric Mann-Whitney U test. *p<0.05
Measurement of plaque and gingiva indices in both groups.
| Variable | CF | S-ECC | p-value1 |
|---|
| Mean±sd | Median | Mean±sd | Median |
|---|
| Plaque score | 1.21±0.072 | 1.17 | 1.74±0.083 | 1.67 | 0.001* |
| Gingival score | 0.17±0.060 | 0.00 | 1.34±0.339 | 1.00 | 0.001* |
1Nonparametric Mann-Whitney U test. *p<0.05
Correlation of clinical parameters and microbial finding in both groups.
| Clinical characteristics | S. sanguinis | S. mutans (saliva) | S. Mutans (Plaque) | S. sobrinus (plaque) |
|---|
| CC | p-value1 | CC | p-value1 | CC | p-value1 | CC | p-value1 |
|---|
| dmft | -0.34 | 0.001* | 0.43 | 0.001* | 0.337 | <0.001* | 0.732 | <0.001* |
| Age | 0.052 | 0.572 | -0.208 | 0.023 | -0.256 | 0.005* | 0.037 | 0.691 |
| PI | 0.272 | 0.003* | 0.62 | 0.001* | 0.079 | 0.391 | 0.348 | <0.001* |
| GI | -0.25 | 0.006* | 0.323 | 0.001* | 0.285 | 0.002* | -0.072 | 0.432 |
1Spearman correlation. *p<0.05
CC: Correlation coefficient
Discussion
This is the first study in Thai children which determined the amount of S. mutans, S. sobrinus and S. sanguinis from both dental plaque and saliva. Previous studies reported that S. mutans and the ratio of S. mutans to total bacteria were higher in S-ECC [8,15]. Another research in Thai children found that the severity of ECC correlated with high levels of S. mutans and S. sobrinus [29]. Corresponding with this study, we found that S-ECC children had higher levels of S. mutans, S. sobrinus, S. mutans/total bacteria and S. sobrinus/total bacteria than caries-free children. From previous studies, S. mutans was detected in high level while S. sobrinus was detected in lower level in S-ECC than S. mutans [7-10]. Martinez-Martinez RE et al., compared the distribution of oral streptococci from saliva of caries-free and caries-affected Mexican children. They reported that S. mutans was identified in 80% of the caries-affected while S. sobrinus was detected 70% in the caries-affected children [16]. Contrast to the previous study in Thai Children, present study indicated that when subjects were infected by both S.mutans and S.sobrinus, the caries prevalence was the same as subjects infected by S.sobrinus alone. There might be a high possibility that S.sobrinus was not found without the presence of S. mutans [7].
In this study, it was found that in S-ECC dental plaque, mean level of S.mutans was higher than S.sobrinus which corresponds to other studies [7-10]. However, previous study reported that the acidogenicity of S. sobrinus was greater than S. mutans [30]. Several studies in children found that increasing S.sobrinus colonisation in dental plaque was correlated with aggravated caries activity as well as that in saliva [8,31,32]. From previous study, there was high baseline count of S.sobrinus in children with recurrent caries. This indicated that besides S.mutans, S.sobrinus can be used as a part of caries risk assessment, especially in high-risk group [33].
There are numerous studies about bacteria composition in dental plaque of caries-free and caries-active children. Li Y et al., analysed the diversity of microorganism in different caries status. They found that the diversity of microorganism in caries-free group was higher than that of caries active [34]. Other study reported that bacterial diversity was decreased when caries progress from healthy to active lesion [35]. Correspond to the present study, which found that total bacteria level in dental plaque of caries-free is higher than those in S-ECC group. From ecological plaque hypothesis, dental caries is the result from imbalance of oral micoorganisms. This results in shifting of bacterial community from healthy bacteria to acid tolerant bacteria. The new condition promotes the proliferation of cariogenic bacteria but decreases the diversity of the plaque community [34]. It can imply that the higher diversity of bacteria in caries-free condition can suppress the condition promoting dental caries [34,35].
Choi EJ and colleagues found that the level of S.mutans showed low correlation with dmfs scores while the ratio of S.mutans to total bacteria, the level of S.sobrinus and the ratio of S.sobrinus to total bacteria had positive correlation with dmfs score [8]. In this study, we found that the level of S.mutans, the ratio of S.mutans to total bacteria and the ratio of S.sobrinus to total bacteria in mature plaque were positively correlated with dmft scores. Various studies reported that the presence of dental plaque was associated with dental caries, but some studies did not [36,37]. We found that the level of S. sobrinus and the ratio of S. sobrinus to total bacteria in mature plaque was positively correlated with plaque index. Law V and Seow WK reported that S.mutans and S.sobrinus infection was correlated with dental plaque amount [37]. Prevalence of S.mutans was higher in children with visible plaque than that of plaque-free group [38-40]. This study showed that the level of S.mutans and the proportion of S.mutans to total bacteria in dental plaque correlated with gingival index. This is similar to previous study which reported that the prevalence of S.mutans was positive correlated with visible dental plaque, gingival inflammation and bleeding [38]. Beighton D et al., also reported that level of S.mutans and S.sobrinus in saliva showed correlation with gingival scores [41].
For S.sanguinis, in this study, its level in saliva was different and higher in caries-free group. Previous study from Thai children also demonstrated that S.sanguinis level was higher in caries-free group when compared with those of S-ECC [6]. Moreover, S.sanguinis level was inversely correlated with dmft scores. This study corresponded with previous studies [6,42,43].
There were many researchers who studied the interaction between oral streptococci in saliva and dental plaque, few studies reported the antagonism action between S.sanguinis and S.mutans. In this study, S. mutans level was higher in S-ECC groups than those of caries-free group which corresponded to previous studies that S. mutans is the main cariogenic microorganism and they might inhibit the growth of S. sanguinis [44,45]. Kreth J et al., investigated the molecule mechanism of the antagonism interaction between S. mutans and S. sanguinis, they reported that S. sanguinis could inhibit the growth of S. mutans by hydrogen peroxide (H2O2) whereas mutacins produced by S. mutans are involved in S. sanguinis growth inhibition and the mutacins mutants had reduced ability to inhibit the growth of S. sanguinis [45]. In this study, we found positive correlation between level of S. sanguinis and dmft score. S. sanguinis might also be used as an indicator with S. mutans for predicting dental caries. From previous studies, they also suggested that the ratio of S. mutans/S. sanguinis can indicate the risk of caries [46,47]. Loesche WJ and Syed SA reported that the percentages of S. sanguinis decreased as the plaque score increased [23]. The proportions of S. sanguinis steadily declined as the gingivitis developed [44]. Corresponded with this study where plaque and gingival scores were higher in S-ECC group. Furthermore, S. sanguinis was inversed correlated with plaque and gingival indices [44].
In the present study, we found the relationships between caries status and related factors from questionnaire. Several studies reported that ECC are commonly found in children with low economic status [42,43,46]. Sarumathi T et al., found that low socioeconomic children had higher risk of developing dental caries than those in middle or high socioeconomic status [43]. The result of this study found that caries-free children had greater proportion of high household income than S-ECC group. Correlation between caries status and parent’s education level has been reported. Various studies suggested that ECC was commonly found in children whose guardians have low literacy level [42,46]. Sarumathi T et al., study found that the children whose parents had high educational level had lower caries prevalence similar to this study [43].
The results of this study found that S-ECC group had more smokers in the house than caries-free group. Household tobacco smoke increased risk of ECC [44,45]. Infection is caused by chemical toxin in tobacco which suppresses or modulates immune system [44]. In-vitro study mentioned that phagocytes acitivities of neutrophils and monocytes were inhibited by nicotine [44]. Vitamin C in blood levels of smokers and children with second-hand smoking were decreased [47-49]. This condition prefers the growth of S. mutans [49]. Second-hand smoked children received the same toxins as the active smoker but in lower doses, so they might receive the same oral health affect as those in active smoking. Previous study reported that the levels of S. mutans were increased in active smoking [50]. Same as the study from Sakki T et al., which mentioned that nicotine in tobacco increased the growth of S. mutans and it was transmitted from guardians to their children [47]. Another possible factor is that active smoking parent might have poor dietary behaviour, unhealthy lifestyle and lack of oral health awareness. This condition might affect their children’s oral health.
In this study, we found that S-ECC group had frequent consumption of food in every types of dietary except fruit and protein. Various studies mentioned about the correlation between high frequency of food and beverage consumption and dental caries [51,52]. The frequency and amount of beverage consuming in S-ECC children were greater than that of caries-free children [53].
Numerous studies found the association between bottle feeding and dental caries [3-5]. In this study, we found that S-ECC had more duration of bottle feeding than caries-free children and S-ECC was associated with history of sleeping with bottle or breast feeding. Furthermore, S-ECC children had history of sleeping with bottle or breast feeding more than caries-free children. The relation between bottle sleeping and caries was found [3]. Du M et al., study stated that bottle-fed children were five times higher risks in developing early childhood caries than breast-fed children [54]. There were various advantages from breast-feeding. Infant received nutrition and immunological protection through human milk. In contrast, the association between breastfeeding and dental caries was inconclusive. From systematic review of epidemiological evidence mentioned that children who breast-fed longer than 1 year or at night might had higher prevalence of dental caries [55]. Dissimilarly, other systematic review stated that there was no scientific evidence about the cariogenicity of human milk [56].
Limitation(s)
The cross-sectional design of the study limit in finding out the predicting factor in questionnaires. In addition, this study used the subjects from the district that have similar socioeconomics and demographic information. The result need to be interpreted carefully so as to generalise the representatives of Thai children.
Conclusion(s)
S.mutans and S.sobrinus were associated with S-ECC while S.sanguinis was associated with caries-free group. Socioeconomics and children’s oral hygiene care and diet were important factors which were found associated with S-ECC.
Author declaration: This study was partially presented at the IADR Meeting 2018 (https://iadr2018.zerista.com/event/member/491734).
[1]. American Academy of Paediatric DentistryGuideline on caries-risk assessment and management for infants, children, and adolescentsPaediatr Dent (Reference Manual) 2012-2013 34:118-25. [Google Scholar]
[2]. Dental Health division MoPH, Thailand. The 7th National dental health status survey (2012). 2013 [Google Scholar]
[3]. Berkowitz RJ, Causes, treatment and prevention of early childhood caries: A microbiologic perspectiveJournal of Can Assoc 2003 69(5):304-07. [Google Scholar]
[4]. Tanzer JM, Livingston J, Thompson AM, The microbiology of primary dental caries in humansJ Dent Educ 2001 65(10):1028-37. [Google Scholar]
[5]. Tinanoff N, O’Sullivan DM, Early childhood caries: overview and recent findingsPaediatric Dentistry 1997 19(1):12-16. [Google Scholar]
[6]. Mitrakul K, Vongsawan K, Sriutai A, Thosathan W, Association between S. mutans and S. sanguinis in severe early childhood caries and caries-free children: A quantitative real time pcr analysisJ Clin Paediatr Dent 2016 40(4):281-89.10.17796/1053-4628-40.4.28127471805 [Google Scholar] [CrossRef] [PubMed]
[7]. Mitrakul K, Asavanund Y, Vongsavan K, Prevalence of five biofilm-related oral streptococci species from plaqueJ Clin Paediatr Dent 2011 36(2):161-66.10.17796/jcpd.36.2.d7r750u227j8581322524078 [Google Scholar] [CrossRef] [PubMed]
[8]. Choi EJ, Lee SH, Kim YJ, Quantitative real-time polymerase chain reaction for Streptococcus mutans and Streptococcus sobrinus in dental plaque samples and its association with early childhood cariesInt J Paediatr Dent 2009 19(2):141-47.10.1111/j.1365-263X.2008.00942.x19250396 [Google Scholar] [CrossRef] [PubMed]
[9]. Ahmady K, Marsh PD, Newman HN, Bulman JS, Distribution of Streptococcus mutans and Streptococcus sobrinus at sub-sites in human approximal dental plaqueCaries Res 1993 27(2):135-39.10.1159/0002615318319257 [Google Scholar] [CrossRef] [PubMed]
[10]. De Soet JJ, van Loveren C, Lammens AJ, Pavicić MJ, Homburg CH, ten Cate JM, Differences in cariogenicity between fresh isolates of Streptococcus sobrinus and Streptococcus mutans.Caries Res 1991 25(2):116-22.10.1159/0002613531829395 [Google Scholar] [CrossRef] [PubMed]
[11]. Igarashi T, Yamamoto A, Goto N, PCR for detection and identification of Streptococcus sobrinusJ Med Microbiol 2000 49(12):1069-74.10.1099/0022-1317-49-12-106911129717 [Google Scholar] [CrossRef] [PubMed]
[12]. Okada M, Soda Y, Hayashi F, Doi T, Suzuki J, Miura K, PCR detection of Streptococcus mutans and S. sobrinus in dental plaque samples from Japanese pre school childrenJ Med Microbiol 2002 51(5):443-47.10.1099/0022-1317-51-5-44311990497 [Google Scholar] [CrossRef] [PubMed]
[13]. Okada M, Soda Y, Hayashi F, Doi T, Suzuki J, Miura K, Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in pre-school childrenJ Med Microbiol 2005 54(7):661-65.10.1099/jmm.0.46069-015947431 [Google Scholar] [CrossRef] [PubMed]
[14]. Hirose H, Hirose K, Isogai E, Miura H, Ueda I, Close association between Streptococcus sobrinus in the saliva of young children and smooth-surface caries incrementCaries Res 1993 27(4):292-97.10.1159/0002615538402804 [Google Scholar] [CrossRef] [PubMed]
[15]. Hata S, Hata H, Miyasawa-Hori H, Kudo A, Mayanagi H, Quantitative detection of Streptococcus mutans in the dental plaque of Japanese preschool children by real-time PCRLett Appl Microbiol 2006 42(2):127-31.10.1111/j.1472-765X.2005.01821.x16441376 [Google Scholar] [CrossRef] [PubMed]
[16]. Martinez-Martinez RE, Fujiwara T, Patino-Marin N, Hoshino T, Wilson M, Loyola Rodriguez JP, Comparison of oral streptococci biofilm in caries-free and caries-affected preschool Mexican childrenActa Odontol Latinoam 2012 25(1):27-32. [Google Scholar]
[17]. Caufield PW, Dasanayake AP, Li Y, Pan Y, Hsu J, Hardin JM, Natural history of Streptococcus sanguinis in the oral cavity of infants: Evidence for a discrete window of infectivityInfect Immun 2000 68(7):4018-23.10.1128/IAI.68.7.4018-4023.200010858217 [Google Scholar] [CrossRef] [PubMed]
[18]. Kreth J, Zhang Y, Herzberg MC, Streptococcal Antagonism in Oral Biofilms Streptococcus sanguinius and Streptococcus gordonii Interference with Streptococcus mutansJ Bacteriology 2008 190(13):4632-40.10.1128/JB.00276-0818441055 [Google Scholar] [CrossRef] [PubMed]
[19]. Ge Y, Caufield PW, Fisch S, Li Y, Streptococcus mutans and Streptococcus sanguinis colonization correlated with caries experience in childrenCaries Res 2008 42(6):444-48.10.1159/00015960818832831 [Google Scholar] [CrossRef] [PubMed]
[20]. American Academy of Paediatric DentistryPolicy on early childhood caries (ECC): classifications, consequences, and preventive strategiesPaediatr Dent 2012-2013 34:50-52. [Google Scholar]
[21]. Greene JC, Vermillion JR, The simplified oral hygiene indexJ Am Dent Assoc 1964 68:713-16.10.14219/jada.archive.1964.003414076341 [Google Scholar] [CrossRef] [PubMed]
[22]. Lobene RR, Weatherford T, Ross NM, Lamm RA, Menaker L, A modified gingival index for use in clinical trialClin Prev Dent 1986 8(1):03-06. [Google Scholar]
[23]. Loesche WJ, Syed SA, Bacteriology of human experimental gingivitis: Effect of plaque and gingivitis scoreInfect Immun 1978 21(3):830-39.10.1128/IAI.21.3.830-839.1978 [Google Scholar] [CrossRef]
[24]. Sinsimer D, Leekha S, Park S, Marras SA, Koreen L, Willey B, Use of a multiplex molecular beacon platform for rapid detection of methicillin and vancomycin resistance in Staphylococcus aureusJ Clin Microbiol 2005 43(9):4585-91.10.1128/JCM.43.9.4585-4591.200516145111 [Google Scholar] [CrossRef] [PubMed]
[25]. Yano A, Kaneko N, Ida H, Yamaguchi T, Hanada N, Real-time PCR for quantification of Streptococcus mutansFEMS Microbiol Lett 2002 217(1):23-30.10.1111/j.1574-6968.2002.tb11451.x12445641 [Google Scholar] [CrossRef] [PubMed]
[26]. Sato T, Matsuyama J, Kumagai T, Mayanagi G, Yamaura M, Washio N, Nested PCR for detection of mutans streptococci in dental plaqueLett Appl Microbiol 2003 37(1):66-69.10.1046/j.1472-765X.2003.01359.x12803559 [Google Scholar] [CrossRef] [PubMed]
[27]. Hoshino T, Kawaguchi M, Shimizu N, Hoshino N, Ooshima T, Fujiwara T, PCR detection and identification of oral streptococci in saliva samples using gtf genesDiagn Microbiol Infect Dis 2004 48(3):195-99.10.1016/j.diagmicrobio.2003.10.00215023429 [Google Scholar] [CrossRef] [PubMed]
[28]. Mitrakul K, Chanvitan S, Jeamset A, Vongsawan K, Quantitative analysis of S. mutans, Lactobacillus and Bifidobacterium found in initial and mature plaques in Thai children with early childhood cariesEur Arch Paediatr Dent 2017 18(4):251-61.10.1007/s40368-017-0295-728721668 [Google Scholar] [CrossRef] [PubMed]
[29]. Saraithong P, Pattanaporn K, Chen Z, Khongkhunthian S, Laohapensang P, Chhun N, Streptococcus mutans and Streptococcus sobrinus colonization and caries experience in 3- and 5-year-old Thai childrenClin Oral Investig 2015 19(8):1955-64.10.1007/s00784-015-1437-025753978 [Google Scholar] [CrossRef] [PubMed]
[30]. Kohler B, Birkhed D, Olsson S, Acid production by human strains of Streptococcus mutans and Streptococcus sobrinusCaries Res 1995 29(5):402-06.10.1159/0002620998521443 [Google Scholar] [CrossRef] [PubMed]
[31]. Nurelhuda NM, Al-Haroni M, Trovik TA, Bakken V, Caries experience and quantification of Streptococcus mutans and Streptococcus sobrinus in saliva of Sudanese school childrenCaries Res 2010 44(4):402-07.10.1159/00031666420714152 [Google Scholar] [CrossRef] [PubMed]
[32]. Rupf S, Merte K, Eschrich K, Kneist S, Streptococcus sobrinus in children and its influence on caries activityEur Arch Paediatr Dent 2006 1(1):17-22.10.1007/BF0332081017140523 [Google Scholar] [CrossRef] [PubMed]
[33]. Tanner ACR, Mathney JMJ, Kent RL, Chalmers NI, Hughes CV, Loo CY, Cultivable Anaerobic Microbiota of Severe Early Childhood CariesJ Clin Microbiol 2011 49(4):1464-74.10.1128/JCM.02427-1021289150 [Google Scholar] [CrossRef] [PubMed]
[34]. Li Y, Ku CYS, Xu J, Saxena D, Caufield PW, Survey of oral microbial diversity using PCR based denaturing gradient gel electrophoresisJ Den Res 2005 84(6):559-64.0.1177/15440591050840061415914595 [Google Scholar] [CrossRef] [PubMed]
[35]. Jiang W, Jiang Y, Li C, Liang J, Investigation of supragingival plaque microbiota in different caries status of Chinese preschool children by denaturing gradient gel electrophoresisMicrob Ecol 2011 61(2):342-52.10.1007/s00248-010-9753-z20927511 [Google Scholar] [CrossRef] [PubMed]
[36]. Karjalainen S, Soderling E, Sewon L, Lapinleimu H, Simell O, A prospective study on sucrose consumption, visible plaque and caries in children from 3 to 6 years of ageCommunity Dent Oral Epidemiol 2001 29(2):136-42.10.1034/j.1600-0528.2001.290208.x11300173 [Google Scholar] [CrossRef] [PubMed]
[37]. Law V, Seow WK, A longitudinal controlled study of factors associated with mutans streptococci infection and caries lesion initiation in children 21 to 72-months-oldPaediatr Dent 2006 28(1):58-65. [Google Scholar]
[38]. Kanasi E, Johansson I, Lu SC, Kressin NR, Nunn ME, Kent R Jr, Microbial risk markers for childhood caries in paediatricians’ officesJ Den Res 2010 89(4):378-83.10.1177/002203450936001020164496 [Google Scholar] [CrossRef] [PubMed]
[39]. Wennhall I, Matsson L, Schroder U, Twetman S, Caries prevalence in 3-year-old children living in a low socio-economic multicultural urban area in southern SwedenSwed Dent J 2002 26(4):167-72. [Google Scholar]
[40]. Mohebbi SZ, Virtanen JI, Vahid-Golpayegani M, Vehkalahti MM, Early childhood caries and dental plaque among 1-3-year-olds in Tehran, IranJ Indian Soc Pedod Prev Dent 2006 24(4):177-81.10.4103/0970-4388.2807317183180 [Google Scholar] [CrossRef] [PubMed]
[41]. Beighton D, Adamson AJ, Rugg-Gunn AJ, Associations between dietary intake, dental caries experience and salivary bacterial levels in 12-year-old English school childrenArch Oral Biol 1996 41(3):271-80.10.1016/0003-9969(96)84555-9 [Google Scholar] [CrossRef]
[42]. Shulman JD, Is there an association between low birth weight and caries in the primary dentition?Caries Res 2005 39(3):161-67.10.1159/00008479215914975 [Google Scholar] [CrossRef] [PubMed]
[43]. Sarumathi T, Saravana Kumar B, Hemalatha VT, Aarthi NV, Manjulata D, Prevalence, severity and associated factors of dental caries in 3-6-year-old childrenJ Clin Diagn Res 2013 7(8):1789-92. [Google Scholar]
[44]. Jose T, Thomas A, Pidamale R, Mhambrey S, Shetty SB, Correlation between C.albicans, S.mutans, S.sanguinis and Lactobacillus in ECC, S-ECC and caries free childrenInternational Journal of Recent Scientific Research 2014 5(2):352-56. [Google Scholar]
[45]. Kreth J, Merritt J, Shi W, Qi F, Competition and Coexistence between Streptococcus mutans and Streptococcus sanguinis in the Dental BiofilmJ Bacterio 2005 187(21):7193-203.10.1128/JB.187.21.7193-7203.200516237003 [Google Scholar] [CrossRef] [PubMed]
[46]. Johansson I, Holgerson PL, Kressin NR, Nunn ME, Tanner AC, Snacking habits and caries in young childrenCaries Res 2010 44(5):421-30.10.1159/00031856920720422 [Google Scholar] [CrossRef] [PubMed]
[47]. Aligne CA, Moss ME, Auinger P, Weitzman M, Association of paediatric dental caries with passive smokingJAMA 2003 289(10):1258-64.10.1001/jama.289.10.125812633187 [Google Scholar] [CrossRef] [PubMed]
[48]. Hanioka T, Ojima M, Tanaka K, Yamanoto M, Does secondhand smoke affect the development of dental caries in children? A systematic reviewInt J Environ Res Public Health 2011 8(5):1503-19.10.3390/ijerph805150321655133 [Google Scholar] [CrossRef] [PubMed]
[49]. Väänänen MK, Markkanen HA, Tuovinen VJ, Kulla AM, Karinpää AM, Luoma H, Dental caries and mutans streptococci in relation to plasma ascorbic acidScand. J. Dent. Res 1994 102(2):103-08.10.1111/j.1600-0722.1994.tb01163.x8016554 [Google Scholar] [CrossRef] [PubMed]
[50]. Sakki T, Knuuttila M, Controlled study of the association of smoking with lactobacilli, mutans streptococci and yeasts in salivaEur J Oral Sci 1996 104(5-6):619-22.10.1111/j.1600-0722.1996.tb00151.x9021335 [Google Scholar] [CrossRef] [PubMed]
[51]. Palmer CA, Kent Jr. R, Loo CY, Hughes CV, Stutius E, Pradhan N, Diet and caries-associated bacteria in severe early childhood cariesJ Dent Res 2010 89(11):1224-129.10.1177/002203451037654320858780 [Google Scholar] [CrossRef] [PubMed]
[52]. Nakayama Y, Mori M, Association between nocturnal breastfeeding and snacking habits and the risk of early childhood caries in 18- to 23-month-old Japanese childrenJ epidemiol 2015 25(2):142-47.10.2188/jea.JE2014009725721070 [Google Scholar] [CrossRef] [PubMed]
[53]. Milgrom P, Riedy CA, Weinstein P, Tanner AC, Manibusan L, Bruss J, Dental caries and its relationship to bacterial infection, hypoplasia, diet, and oral hygiene in 6- to 36 month-old childrenCommunity Dent Oral Epidemiol 2000 28(4):295-306.10.1034/j.1600-0528.2000.280408.x10901409 [Google Scholar] [CrossRef] [PubMed]
[54]. Du M, Bian Z, Guo L, Holt R, Champion J, Bedi R, Caries patterns and their relationship to infant feeding and socioeconomic status in 2-4-years-old Chinese childrenInt Dent J 2000 50(6):385-89.10.1111/j.1875-595X.2000.tb00573.x11197198 [Google Scholar] [CrossRef] [PubMed]
[55]. Valaitis R, Hesch R, Passarelli C, Sheehan D, Sinton J, A systematic review of the relationship between breastfeeding and early childhood cariesCanadian Journal of Public Health 2000 91(6):411-17.10.1007/BF0340481911200729 [Google Scholar] [CrossRef] [PubMed]
[56]. Mohebbi SZ, Virtanen JI, Vahid-Golpayegani M, Vehkalahti MM, Feeding habits as determinants of early childhood caries in a population where prolonged breastfeeding is the normCommunity Dent Oral Epidemiol 2008 36(4):363-69.10.1111/j.1600-0528.2007.00408.x19145723 [Google Scholar] [CrossRef] [PubMed]