Unusual Presentation of Primary Ovarian Carcinoid Tumours with Low-to-Moderate Proliferative Potential
Supriya Mehrotra1, Divya Saxena2, AK Kapoor3, Deepika Chaturvedi4, Rajesh Kumar Srivastava5
1 Pathologist, Department of Pathology, RML Mehrotra Pathology Ltd., Lucknow, Uttar Pradesh, India.
2 Pathologist, Department of Pathology, RML Mehrotra Pathology Ltd., Lucknow, Uttar Pradesh, India.
3 Pathologist, Department of Pathology, RML Mehrotra Pathology Ltd., Lucknow, Uttar Pradesh, India.
4 Technician, Department of Pathology, RML Mehrotra Pathology Ltd., Lucknow, Uttar Pradesh, India.
5 Senior Technologist, Department of Pathology, RML Mehrotra Pathology Ltd., Lucknow, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. AK Kapoor, Pathologist, Department of Pathology, RML Mehrotra Pathology Ltd., Nirala Nagar, Lucknow-226020, Uttar Pradesh, India.
E-mail: drashokkapoor2016@gmail.com
Primary Ovarian Carcinoid Tumour (OCT) are rare benign neuroendocrine neoplasms. Herewith, two cases of ovarian tumour has been described. In case 1, patient was a 63-year-old postmenopausal woman with vague abdominal pain. On examination, she had right pelvic tumour. Right salpingo-oophorectomy was performed. Cut surface showed solid, yellowish-white tumour tissue. Microscopically, tumour showed adenomatous, insular and trabecular patterns. Sheets of tumour cells were separated by fibrovascular septa. Tumour consisted of proliferated epithelial cells showing mild nuclear pleomoprhism. Nucleomegaly and capsular invasion were also seen. Carcinoid tumour cells may convert a silver salt to metallic silver (argentaffin reaction). Imunohistochemical (IHC) staining of tumour revealed strong positivity for pancytokeratin and chromogranin A. Mild reaction with anti-neuron-specific enolase antibody was seen. Ki-67 labelling index of 6% suggested moderate proliferative potential of current tumour. Caudal type home box transcription factor 2 (CDX2) non-reactivity suggested it to be a primary neoplasm. In case 2, the patient was 60-year-old. She had left ovarian tumour. Tumour formed solid masses (insular) and nests forming rosettes. IHC showed strong reactivity with anti-chromogranin A and mild reactivity with anti-neuron-specific enolase. Anti-Ki-67 did not stain most of tumour cells (Ki-67 index 1%). Anti-CDX2 failed to stain tumour cells.
Chromogranin A, Low proliferative potential, Neurosecretory neoplasm, Neuron-specific enolase
Case Report
Case 1
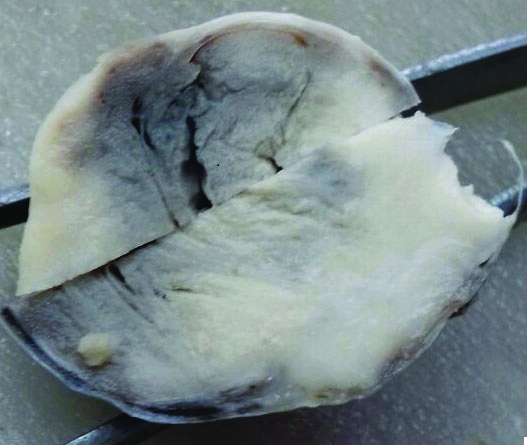
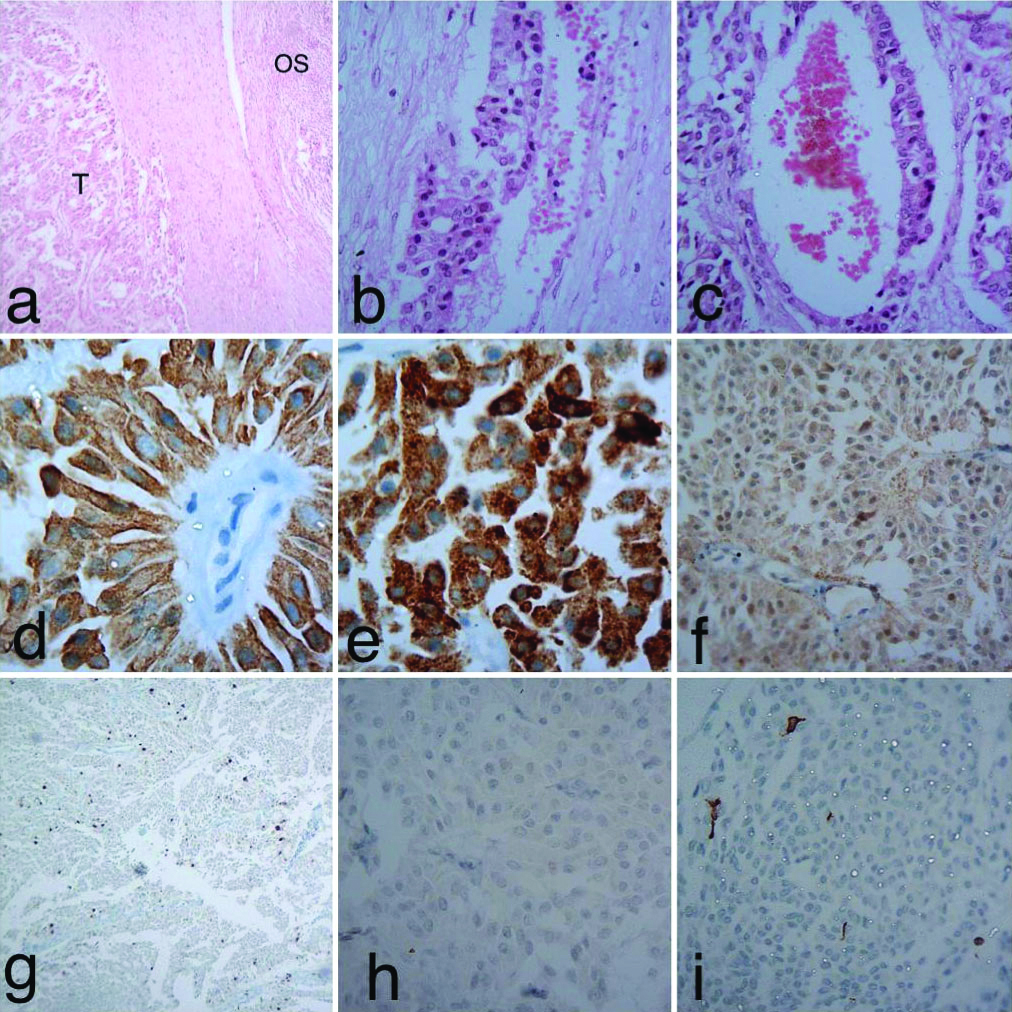
A 63-year-old female complained of vague pain in right lower abdomen since four months. On palpation of abdomen, a right pelvic tumour was felt. Approximately, tumour measured 6 cm in diameter She was operated and right salpingo-oophorectomy was done. Cut surface showed a solid, yellowish-white clearly demarcated tumour [Table/Fig-1]. It measured 6×5×5 cm. Tumour was encapsulated. Microscopically, thick fibrovascular capsule separated tumour from ovarian stroma [Table/Fig-2a]. Capsular invasion was also seen [Table/Fig-2b]. Tumour consisted of adenomatous, insular and trabecular components. Adenomatous element consisted of irregular medium-sized glands [Table/Fig-2c]. Adenocarcinoid component may have higher rate of aggressive behaviour than typical carcinoid. Papillary structures with fibrovascular core were also seen. Tumour consisted of epithelial cells having round to oval nuclei showing nucleomegaly. Mild nuclear pleomorphism was also seen. Islands of tumour cells were separated by fibrovascular septa. The patient was, provisionally diagnosed as a case of low-grade carcinoma or adenoma. Fallopian tube showed normal morphology. IHC examination revealed strong positivity for pancytokeratin [Table/Fig-2d] and chromogranin A, a neurosecretory marker [Table/Fig-2e]. Staining of tumour cells with anti-neuron-specific enolase antibody revealed weak positivity for this marker [Table/Fig-2f]. Nuclear staining revealed Ki-67 index of 6%, suggesting relatively moderate proliferative potential of tumour [Table/Fig-2g]. Caudal type homeobox transcription factor 2 (CDX2) antigen negativity of current tumour suggested it to be a primary neoplasm [Table/Fig-2h]. Metastatic bowel carcinoids are known to be CDX2 positive. In addition, anti-vimentin antibody did not stain tumour cells [Table/Fig-2i]. Differential diagnosis of adenoma was ruled out on the basis of IHC findings. She was finally diagnosed as a case of carcinoid tumour of right ovary. The patient could not be followed further.
Cut surface of tumour shows solid yellowish-white appearance. Tumour appeared to be encapsulated.
a) Shows tumour, thick fibrous tissue and ovarian tissue (H&E×40); b) Shows capsular invasion by tumour tissue (H&E×100); c) Shows adenomatous component of tumour (H&E×100); d) IHC examination showed strong positivity for cytokeratin (×1000); e) Shows strong positivity for chromogranin A (×1000); f) Shows weak reaction with anti-neuron-specific enolase (×400); g) Shows moderate nuclear staining for Ki-67 (×100); h) Anti-CDX2 antibody did not stain tumour cells (×400); i) Anti-vimentin antibody failed to stain tumour cells (×400); Abbreviations: OS=ovarian stroma, T=tumour tissue.
Case 2
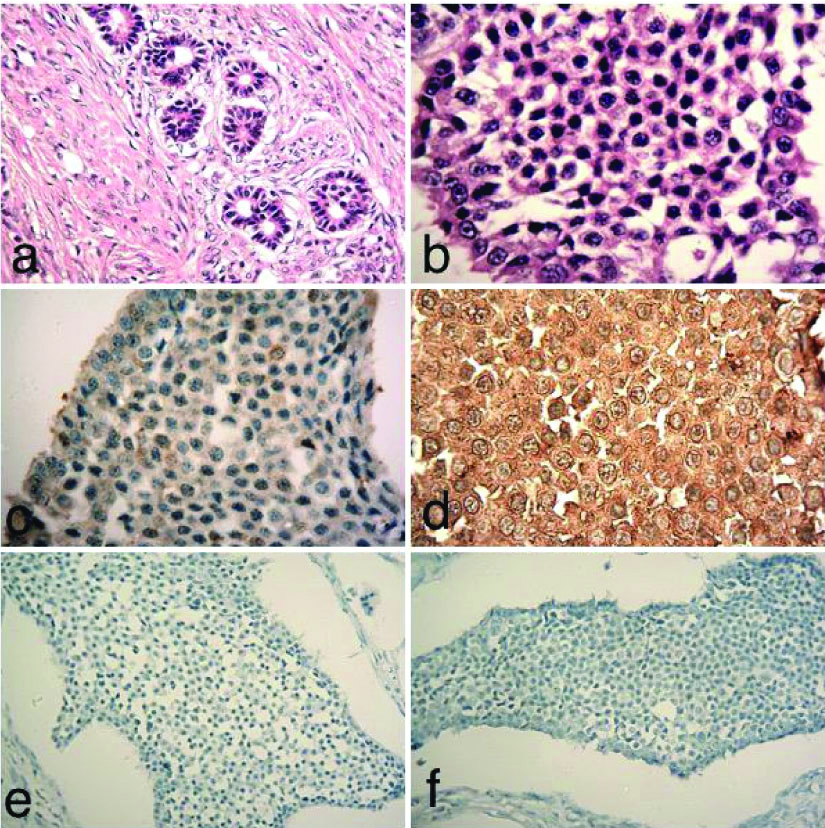
A 60-year-old female complained of pain in left side of abdomen since three months. Later, she was operated and tumour was resected. Tumour measured 6x6x5 cm. Provisionally, it was diagnosed as a benign neoplasm. Microscopically, the tumour consisted of uniform small round cells. At places, tumour cells formed solid masses (insular) and nests forming rosettes. Abundant fibrocollagenous tissue formation (desmoplasia) was seen around the tumour tissue [Table/Fig-3a]. Retraction of tumour from stroma was also evident. Tumour cells showed hyperchromatism and mild nuclear pleomorphism [Table/Fig-3b]. Haematoxylin-Eosin (HE) examination suggested it to be an adenocarcinoma. However, IHC showed mild reactivity with anti-neuron-specific enolase antibody [Table/Fig-3c]. In addition, anti-chromogranin A showed strong reactivity with tumour cells [Table/Fig-3d]. Anti-CDX2 antibody did not stain tumour cells [Table/Fig-3e]. IHC findings were suggestive of a primary OCT. Anti-Ki-67 antibody failed to stain most of the tumour cells (Ki-67 index 1%) suggesting low proliferative potential of tumour [Table/Fig-3f]. Postoperative period was satisfactory. The patient appeared cured following resection of tumour.
a) Shows tumour tissue, consisting of uniform round cells forming rosettes. Fibroblastic proliferation around tumour tissue was also seen (H&E×400); b) Tumour cells showed mild nuclear pleomorphism (H&E×1000); c) IHC showed mild reactivity with anti-neuron-specific enolase antibody (×400); d) Strong reactivity was seen with anti-chromogranin A antibody (×1000); e) Anti-CDX2 antibody did not stain tumour cells (×100); f) Anti-Ki-67 antibody did not stain most of tumour cells, suggesting a low-proliferative potential of tumour (×100).
Discussion
Primary carcinoid tumour is a neurosecretory neoplasm with low proliferative potential [1]. Its metastatic potential may be related with size of tumour [2]. It is very rare in ovary. Either it may primarily arise in an ovary or it may be found as metastatic tumours in both the ovaries. Five different morphological patterns may be seen, e.g., insular, trabecular, strumal, adenomatous (mucinous) and mixed strumal and mucinous. Rarely, primary OCT may be accompanied with luteinized stromal cells. Insular carcinoid is relatively more common in west and trabecular more in Asia. Ileal carcinoid tumours may be multicentric [2]. These tumours may also arise in association with multiple endocrine neoplasia type 1. Incidence of primary OCT may vary from 0.3% to 1% of all carcinoid tumours [3]. The prognosis of primary OCT may be excellent whereas metastatic carcinoids have poor outcome. Surgical resection of a primary OCT of insular or trabecular type may be curative. However, behaviour of mucinous carcinoid may be more aggressive. Within the last seven years, we had seven cases of carcinoid tumours; five of these arose from gut (appendix 2, colon 1, hemorrhoid 1 and stomach 1 case). Other two cases including the present cases arose from ovary. Both of the cases presented are different from other published cases of carcinoid tumours. Firstly, both cases presented as primary benign neoplasms; Secondly, in both cases, tumour cells had low-to-moderate proliferative potential. In addition, both the tumours arose from ovaries.
IHC was planned to investigate the reactivity of tumour using CDX2 antibody. In addition, anti-Ki-67 was used to determine the proliferative potential of tumour. Most significant finding of current tumours was double positivity for chromogranin A and neuron-specific enolase [4,5]. Cytokeratin antigen [6] and CD56 antigen [5] positivity have been reported earlier. CDX2 positivity may be useful for distinguishing primary OCT from metastatic bowel carcinoids; bowel carcinoids are CDX2 positive [7]. Rare mitoses and low Ki-67 index of 1% and 6% cells suggested low-to-moderate proliferative potential of both the neoplasms. In another study, tumour cells showed 3 mitoses/10 hpf and Ki-67 index of 17%, suggesting high proliferative activity [5]. Thus, Ki-67 antigen appeared to be a prognostic marker [6]. Benign tumours had significantly lower Ki-67 index when compared with malignant tumours [5]. Growth fraction defined by Ki-67 may correlate with clinical stage of carcinoid tumour. For example, the Ki-67 index was higher in advanced stage tumours. Ki-67 index appeared to be an excellent marker to assess clinical outcome of carcinoid tumour [8]. In another study, expression of Ki-67 was extremely high in group A (>50 labelled nuclei/field); neuroendocrine tumours progressed rapidly with worst outcome [9].
Several ovarian carcinoids may also produce hormones, e.g., serotonin, testosterone, estradiol and insulin [10]. These hormones as well as other unknown tumour-products may produce clinical features suggestive of carcinoid syndrome. Classical symptoms of carcinoid syndrome include episodic flushing, bronchospasm and cyanosis. In addition, carcinoid tumours may produce CA-125. They are capable of producing various amines and peptides, e.g., Pyy. Pyy antigen positivity of tumour cells has been reported with severe constipation [11,12]. Generally, the cut surface of colorectal carcinoid acquires yellow color after formalin fixation. However, the cut surface of current tumours was yellowish-white after formalin treatment. Vimentin negativity for one of the current tumours ruled out the possibility of its origin from a mesenchymal cell. Unusual presentation of carcinoid tumour may occur. For example, primary OCT may occur in a Mature Cystic Teratoma (MCT). Rarely, malignant change may occur, e.g., struma ovarii with a focus of papillary carcinoma, mucinous adenocarcinoma and strumal carcinoid may develop in MCT. Strumal carcinoid may be associated with menopausal symptoms [13]. Right-sided heart failure may develop in carcinoid tumour which may resolve after resection of tumour [14]. The patient with carcinoid tumour may present with vaginal bleeding which may disappear after oophorectomy without recurrence of tumour. Rarely, recurrence of OCT may occur after longterm (>13 years) postsalpingo-oophorectomy [15]. Urinary 5-hydroxy indol acetic acid (5-HIAA), a serotonin degradation product may be present in high concentration in a patient with primary OCT. In addition, mild virilizing features may also develop in OCT [16]. Carcinoid tumour is mainly seen in perimenopausal or postmenopausal women. Infrequently, it may develop in a young woman [17]. Ovarian carcinoids may also be multicentric and atypical [18]. Surgery is the treatment of choice in early stages. Advanced disease may be treated by Everolimus (Everolimus is an mTOR inhibitor). It may change the course of disease [5].
Conclusion(s)
Carcinoid tumours have low-to-moderate proliferative potential. Tumor necrosis, frequent mitosis (3 mitoses/10 hpf) and Ki- 67 index of >16%, appeared to be the poor prognostic markers of these tumours. Other factors such as size of the tumour (>2 cm), adenocarcinoid pattern and capsular invasion are also responsible for poor prognosis. Aneuploidy also appeared to favour metastasis. Most of the patients respond well after resection of the tumour, but rare cases with disseminated tumour may respond to Everolimus, mTOR inhibitor.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: May 18, 2019
Manual Googling: Jan 03, 2020
iThenticate Software: Jan 29, 2020 (2%)
[1]. Talerman A, Carcinoid tumours of the ovaryJ Cancer Res Clinic Oncol 1984 107:125-35.10.1007/BF003993836715397 [Google Scholar] [CrossRef] [PubMed]
[2]. Hamilton SR, Farber JL, Rubin E, The gastrointestinal tractIn ‘Pathology’ 1999 3rd ed:669-755.Rubin E, Farber JL, Lippincott-Raven [Google Scholar]
[3]. Levin MA, Flynn BC, Primary ovarian carcinoid: A rare tumour causing unexpected manifestations in a previously undiagnosed womanAnesth and Analg 2011 112:1158-60.10.1213/ANE.0b013e318214292f21372282 [Google Scholar] [CrossRef] [PubMed]
[4]. Reed NS, Gomez-Garcia E, Gallardo-Rincon D, Barrette B, Baumann K, Friedlander M, Gynecologic cancer intergroup (GCIG) consensus review for carcinoid tumours of the ovaryInternat J of Gynec Cancer 2014 24:S35-41.10.1097/IGC.000000000000026525341578 [Google Scholar] [CrossRef] [PubMed]
[5]. Kaiho-Sakuma M, Toyoshima M, Watanabe M, Toki A, Kameda S, Minato T, Aggressive neuroendocrine tumor of the ovary with multiple metastases treated with everolimus: A case reportGynec Oncol Rep 2018 23:20-22.10.1016/j.gore.2018.01.00229326972 [Google Scholar] [CrossRef] [PubMed]
[6]. Kurupayashi T, Minamikawa T, Nishijima S, Tsuneki I, Tamura M, Yanase T, Primary strumal carcinoid tumour of the ovary with multiple bone and breast metastasesJ Obs Gynaec Res 2010 36:567-71.10.1111/j.1447-0756.2010.01231.x20598039 [Google Scholar] [CrossRef] [PubMed]
[7]. Desouki MM, Lioyd J, Xu H, Cao D, Barner R, Zhao C, CDX2 may be a useful marker to distinguish primary ovarian carcinoid from gastrointestinal metastatic carcinoids to the ovaryHum Pathol 2013 144(11):2536-41.10.1016/j.humpath.2013.06.01424029704 [Google Scholar] [CrossRef] [PubMed]
[8]. Choudhury M, Goyal S, Pujani M, A cytohistological study of Ki-67 expression in ovarian tumoursInd J Pathol Microbiol 2011 54:21-24.10.4103/0377-4929.7731821393871 [Google Scholar] [CrossRef] [PubMed]
[9]. Kimura N, Miura W, Noshiro T, Miura Y, Ookuma T, Nagura H, Ki-67 is an indicator of progression of neuroendocrine tumorsEndoc Pathol 1994 5:223-28.10.1007/BF02921490 [Google Scholar] [CrossRef]
[10]. Morken NH, Majak B, Kahn JA, Insulin producing primary ovarian carcinoid tumourActa Obstet et Gynec Scandinav 2007 86:500-01.10.1080/0001634060061347717486477 [Google Scholar] [CrossRef] [PubMed]
[11]. Shohei M, Eisuke O, Yoshio M, Tatsuo N, Kotaro H, Hiroto N, A case of peptide YY-secreting neuroendocrine tumor of the rectum associated with severe constipationAJSP Rev & Rep 2017 22:275-79. [Google Scholar]
[12]. Kristen M, Laura T, Jorge G, Loyd W, Alan S, Ovarian strumal carcinoid producing peptide YY associated with severe constipation: A case report and review of the literatureInt J Gynec Pathol 2015 34:30-35.10.1097/PGP.000000000000011725473750 [Google Scholar] [CrossRef] [PubMed]
[13]. Ghanbarzadeh N, Nadjafi-Semnani M, Azarkar Z, Haghigh F, Nadjafi-Semnani A, Primary strumal carcinoid tumor of the ovary: A case reportIran J Pathol 2014 9(4):285-90. [Google Scholar]
[14]. Goldman T, Adamson K, Yang E, Resolution of right-sided heart failure symptoms after resection of a primary ovarian carcinoid tumorTexas heart Inst J 2014 41(5):533-36.10.14503/THIJ-13-331425425990 [Google Scholar] [CrossRef] [PubMed]
[15]. Amano Y, Tsukasa MM, Junzo B, Yumiko H, Noriomi Y, Konishi MI, Recurrence of a carcinoid tumour of the ovary 13 years after the primary surgery: A case reportOncol let 2013 6(5):1241-44.10.3892/ol.2013.153024179502 [Google Scholar] [CrossRef] [PubMed]
[16]. Gupta B, Suneja A, Vaid N, Bhatia A, Primary ovarian carcinoid tumour simulating virilizing tumour of the ovary: A rare entityInd J Cancer 2014 51(4):52910.4103/0019-509X.17530226842185 [Google Scholar] [CrossRef] [PubMed]
[17]. Delić R, Mucinous cystadenoma of the ovary with carcinoid tumour in a 23-year-old nulliparous womanJ Obs Gyn 2017 37(4):543-44.10.1080/01443615.2016.127429428286998 [Google Scholar] [CrossRef] [PubMed]
[18]. Williams RM, A light and electron microscopic study of an ovarian and rectal carcinoidHistopathol 1979 3:19-30.10.1111/j.1365-2559.1979.tb02978.x34560 [Google Scholar] [CrossRef] [PubMed]