CD is the second most common autoimmune condition affecting children with T1DM following thyroiditis [1]. CD has higher prevalence of 3-16% in children with T1DM as compared to 0.6-1% prevalence in the general population [2-4]. The concurrence of T1DM and CD can be explained by similar genetic association (HLA DQ 2 and 8), environmental and immunological factors in their pathogenesis [5]. Although the exact role of gluten in the pathogenesis of T1DM is not completely understood, demonstration of increased production of pro-inflammatory cells in T1DM patients when stimulated with wheat protein as well as the protective effect of gluten free diet against development of diabetes in animal models could suggest gluten may have a role in T1DM pathogenesis [6,7]. The causative factor(s) triggers a cascade of immune response producing disease-specific antibodies which result in β cell destruction and intestinal mucosal damage [5].
Interestingly, the vast majority of diabetic children with CD do not have gastrointestinal symptoms at the time of a positive celiac screen [8]. The common symptoms such as food intolerance, food avoidance and mild abdominal discomfort in these children are often thought to be due to glycaemic instability and diabetic gastropathy [9]. However, the co-occurrence of CD in children with T1DM can have major impact on their glycaemic status and growth. Therefore, high index of suspicion is crucial in screening for CD in those with T1DM in order to optimise glycaemic control and growth and to minimise the long term sequelae. Early identification of CD and starting gluten free diet will benefit these children.
This study was done to determine the prevalence of CD in children with T1DM in our institution and to evaluate the manifestations which led to its clinical suspicion.
Materials and Methods
This retrospective study was done in children with T1DM treated in a tertiary care referral centre in Southern India who was screened for CD with serum ATTG, between January 2012 to December 2015. Institutional review board and ethics committee approval (IRB no: 12498) was obtained for the study.
Data was collected from their medical records. The decision to screen for CD was at the discretion of the treating Paediatric endocrinologist. A detailed proforma which included the demographic details, clinical feature which led to CD screening, HbA1C levels, ATTG report, gastroscopy findings and tissue biopsy reports were recorded and analysed.
The following criteria were used for analysis: Serum ATTG assay using AESKULISA Celichek kit which measures both IgA and IgG specific antibodies for CD screening and a titer of more than 15 U/mL was reported as positive. Marsh Celiac classification stages I-III was used for histopathological findings in duodenal biopsy [10]. Glucometer blood sugar of less than 3.9 mmol/L (70 mg/dL) was considered as hypoglycaemia and HbA1C of more than 7.5% was considered as poor glycaemic control [11].
Statistical Analysis
Descriptive statistics (mean, standard deviation) were used to summarise the study variables.
Results
A total of 152 children with T1DM, which included 58 boys and 94 girls, were screened for CD during the study period. The mean age of the children was 9.02 years (1-15.8 years). Out of those screened, 18.4% (n=28) children (16 boys and 12 girls) tested positive for ATTG. The mean age at diagnosis was 7.5 years (1-14 years). Average age of the children at diagnosis of T1DM was 6.3 years, ranging from 11 months to 14 years. Mean HbA1C at the diagnosis of CD in 28 children was 11.47% [Table/Fig-1]. Of the 28 children with positive screen, 50% (n=14) consented for duodenal biopsy and underwent the procedure. Among those 14 children, 78.5% (n=11) had histological changes suggestive of CD. There were seven children with Marsh Stage 3a to 3c changes, four children with Marsh stage I changes in the duodenal biopsy. The remaining three children (21.5%) had duodenal biopsy reported as non-specific chronic duodenitis.
Characteristics of children with positive ATTG.
| Characteristics | Number (n=28) | Percentage (%) |
|---|
| Gender |
| Female | 12 | 42.9% |
| Male | 16 | 57.1% |
| Mean age at diagnosis of T1DM (years) | 6.39±4 | |
| HbA1C (%) |
| At CD diagnosis | 11.56±2.8 | |
| On follow-up | 8.6±0.91 | |
| HbA1c on follow-up (n=16) |
| Improved | 14 | 87.5 |
| Not improved | 2 | 12.5 |
| Follow-up |
| Lost to follow-up | 12 | 42.8 |
| 1 year | 7 | 25.0 |
| 2 years | 2 | 7.2 |
| 5 years | 7 | 25.0 |
| Effect of GFD |
| Growth (mean) (cm/year) | 6.18±1.6 | |
| Improvement of symptoms (n=16) | 14 | 87.5 |
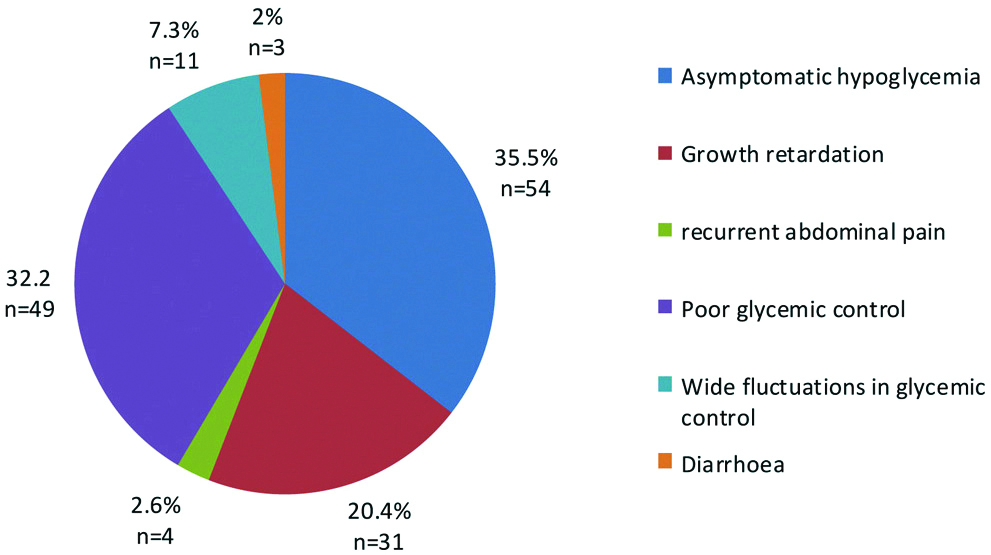
The symptoms which raised clinical suspicion of CD were recurrent unexplained asymptomatic hypoglycaemia (35.5%), poor glycaemic control (32.2%), growth retardation (20.4%) and unexplained widely fluctuating blood sugar levels (7.3%) [Table/Fig-2].
Clinical indications for screening for Celiac disease in T1DM.
Out of the 28 children, 16 children were followed for a period ranging from 1 year to 5 years. Among these children, 14 children (who underwent biopsy and started on GFD) showed improvement in symptoms, growth and glycaemic control. Two children who did not give consent for biopsy were followed-up for a year, they continued to have symptoms but achieved appropriate growth velocity, gastroscopy and mucosal biopsy was advised [Table/Fig-1].
Discussion
This study reports a high prevalence (18.4%) of serology positivity for CD in children with T1DM and ~80% of those who consented for biopsy had mucosal changes. Previously, 11% and 15.5% serology positivity have been reported from North and West India, respectively [3,4]. All children in the North Indian cohort with positive serology showed histopathological changes [3]. Similar to this study, 50% of the screen positive cohort in Western India did not consent for biopsy, however 83% who had biopsy showed mucosal changes. This reiterates the fact that serum ATTG level remains an excellent screening tool for CD.
In general, gastrointestinal symptoms of CD such as abdominal pain and diarrhea may be subtle, atypical or absent in diabetic children [8]. Regional differences exist in the manifestations of CD in children with T1DM both within and outside India [3,4,8]. In the present cohort, unexplained asymptomatic hypoglycaemia, poor glycaemic control and poor growth were the common manifestations.
Early detection is essential, as untreated CD may lead to several complications such as glycaemic instability with risk of hypoglycaemia, increased risk of diabetic retinopathy and nephropathy [12]. Once confirmed, Gluten Free Diet (GFD) with adequate diet education helps in alleviation of symptoms and improvement in mucosal changes [13].
In this study, 11 children with confirmed CD and 3 children with potential CD who had recurrent abdominal pain and poor growth were advised GFD. In a prospective case series, Volta U et al., addressed the management of 61 symptomatic patients with potential CD who had elevated ATTG without villous atrophy on histology. All of them showed clinical improvement with GFD and seroconverted to negative ATTG. This study supports the treatment of symptomatic potential celiac disease with GFD [14].
During follow-up, the children on GFD showed clinical improvement and reduction in HbA1c levels. Varying results have been reported on HbA1C levels on follow-up of T1DM children with CD on GFD. Similar to this study, reduction in HbA1c levels were reported in diabetic children on GFD for CD [15], however, no difference in HbA1C levels on follow-up has also been documented [16].
The International Society for Paediatric and Adolescent Diabetes (ISPAD) recommends screening for CD with ATTG at the time of diagnosis of diabetes and repeat screening at 2 years and 5 years thereafter. More frequent screening is recommended if there is a high clinical suspicion of CD or family history of CD in first degree relative [17]. In children with IgA deficiency, IgG-specific antibody tests (tTG) or endomyseal antibody (EmAIgG) or both are recommended [17]. In resource limited settings, serum IgA levels may be done in ATTG negative individuals before proceeding with further tests. All the children with positive serology should have confirmatory duodenal biopsy. Although some centres perform HLA DQ2 and DQ8 typing to screen for CD in T1DM, its prohibitive cost, limited availability and high prevalence in those with Type I Diabetes limit its use as an effective screening test.
Limitation(s)
Limitations include unavailability of repeat mucosal biopsy and ATTG levels to prove benefit of GFD.
Conclusion(s)
CD frequently co-occurs with T1DM with minimal or absent gastrointestinal symptoms. If universal screening of CD is not possible in resource limited settings, clinicians need to have a high index of suspicion and be aware of its non-gastrointestinal manifestations.