Materials and Methods
Search Strategy
The keywords “curcumin”, “curcuma”, “turmeric extracts”, “oral lichen planus”, “erosive lichen planus”, “pain”, “erythema”, “ulcer” were searched extensively to identify articles published and unpublished in english language till October 2019 using the electronic database such as PubMed, Google scholar, Science direct and Cochrane database. The total number of articles obtained was 1499. Randomised clinical trials; comparative interventions using curcumin and turmeric for the management of OLP were included. In vitro studies, case reports, retrospective studies, animal studies, experimental studies, literature reviews and surveys were excluded from the study.
Screening of articles was on the basis of title and abstract. The articles, which satisfied the inclusion criteria were selected, and the full text were procured.
PICO Evaluation
Population: Patients with Oral lichen planus
Intervention: Turmeric/Curcumin
Comparison: Placebo/Other standardised interventions
Outcome: The primary outcome was the resolution of oral lichen planus in terms of reduction in pain, burning sensation, erythema and ulceration in 4-8 weeks after the treatment has been started. Complete resolution of oral lichen planus after the usage of curcumin for 4-8 weeks would indicate the secondary outcome measure.
Heterogeniety Assessment
Age, sample size, gender wise, research design, formulation of curcumin and control drug dosage and directions, clinical parameters and follow-up.
Methodological Quality Assessment
The methodological quality assessment includes randomization, explanation of allocation concealment, usage of blinded experiment method in order to reduce the risk of bias, estimation of the sample size, comparison of the baseline data and follow-up of the patient after being treated with curcumin. The methodology to be followed has to reduce the risk of bias.
Data Extraction
Study design, number of subjects, age, gender, groups, drug dosage, directions, measurement scales, duration of observation, results, adverse effects.
Results
Selection Criteria
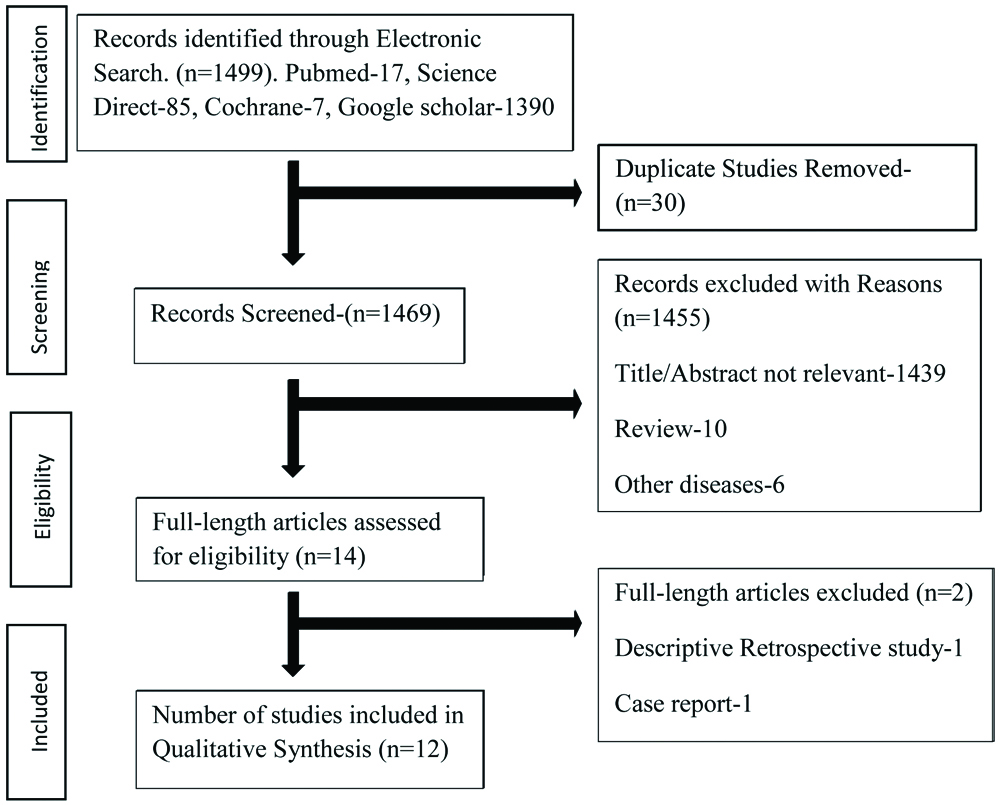
On searching PubMed, Google scholar, Science direct, Cochrane database, and gray literature, a total of 1499 articles were obtained based on the keywords. The number of articles screened by title and abstract was 1469 after removing duplicates. Further 1455 articles were removed. Full length articles assessed for eligibility were 14, out of which 2 articles were removed for not meeting inclusion criteria. A total of 12 articles were finalised for this systematic review based on the entire content of the articles. [Table/Fig-1] shows Prisma flow chart on search strategy.
Prisma Flowchart on search strategy.
Study Design and Duration
Among the selected 12 articles, 3 articles were randomised double blinded placebo controlled trials [11,35,36], 1 article was Randomised double blinded clinical trial [37] while 3 were randomised controlled trials [38-40], 3 were comparative study [41-43], 2 were pilot clinical trials [44,45]. In order to include all prospective studies, 2 articles of curcumin on OLP were excluded due to one being the descriptive retrospective study and one being the case report.
All the patients included in the studies were above 18 years. Duration of studies ranged from 2 weeks to 4 months. Follow-up of studies were done 3 months for 3 studies [37,42,44], 4 weeks for 3 studies [35,36,45], 7 weeks [11], 2 weeks [35], 15 days for 2 studies [39,43], 12 weeks [41], 60 days [40]. [Table/Fig-2] shows the characteristics of included studies.
Characteristics of included articles.
| Year of study and study design | Form of curcumin used | Groups, Dosage and duration | Number of patients | Gender, Age | Results-clinical improvement and pain score | Therapy duration | Adverse effects | Comments | Measurement scales |
|---|
| Singh V et al., [42]Comparative study | Turmeric ointment vs. tulsi ointment in Glycerin base | Group 1-Turmeric ointmentGroup 2-Tulsi ointment twice daily for 3 months | Sample size not given | Not mentioned | Turmeric reduces burning sensation, pain and white lesions. Tulsi Reduces size of lesion and halitosis | Every 2 weeks for 4 months | No adverse effects | Turmeric and Tulsi both are effective in management of OLP. | VAS, NRS, Tgpm |
| Thomas AE et al., [38]Randomised control trial | 0.1% Triamcinolone acetonide oral paste vs. Curcumin oral gel | Group 1-Triamcinolone acetonide oral paste thrice daily in tapering dosesGroup 2-Curcumin oral gel thrice dailyGroup 3-curcumin oral gel six times daily | 75 patients | Male-19Female-5620-70 yrs. | Reduction in burning sensationGroup 1- 77.3%Group 2- 54.4%Group 3- 64.99%Reduction in erythema, ulcerationGroup 1- 67.8%Group 2- 46.6%Group 3- 58.36% | Every 2 weeks for 3 months | No adverse effects | Curcumin can be used as maintenance drug after initial treatment with corticosteroids. | NRS, MOMI |
| Amirchaghmaghi M et al., [36]Randomised double blinded, placebo-controlled trial | Curcumin 500 mg tablets vs. placebo tablets 4 in number.Both Groups received (Mouthwash Dexamethasone 0.5 mg and suspension Nystatin 100,000 Units) | Group 1-Curcumin tablet 2000 mg/dayGroup 2-placebo tablets containing lactose two times a day | 20 OLPCases Group-12, Control-8 | Male-7, Female-13Group 1- 52.7±9.4 yrsGroup 2- 49.4±11.2 yrs. | No significant difference between curcumin and placebo. | 0,2,4 weeks | No adverse effects | No detectable effect of curcumin in the treatment of OLP | VAS, Tgpm |
| Kia SJ et al., [37]Randomised double blinded clinical trial | 0.1% triamcinolone oral paste vs. 5% curcumin oral paste | Group 1-0.1% triamcinolone oral paste Group 2-5% curcumin oral paste. Oral paste was applied three times a day for four weeks | 50 patients | (36=women14=men.Group 1- 52.08 yrsGroup 2-49.24 yrs | Curcumin group-Pain reduction: 36%-complete remission16%-good response24%-poor response24%-no responseTriamcinolone group-Pain reduction:32%-complete remission32%-good response16%-poor response20%-no response | 0,2,4 weeks | Burning sensation, itching, mild swelling and xerostomia, disappeared at the end of the first week of drug consumption | Application of curcumin is suggested due to anti-inflammatory effects, insignificant side-effects. | VAS, Tgpm |
| Singh V et al., [44]Pilot study | Curcumin ointment | Local application twice/day | 10 patients | Not mentioned | Over a span of 4 weeksComplete resolution-90%Marked resolution-10% | 3 months | No adverse effects | Herbal modality of oral lichen planus with no or minimal side effects. | VAS, Tgpm |
| Keshari D et al., [39]Randomised control trial | Curcumin ointment vs. Triamcinolone ointment | Study Group-Topical curcuminControl Group-Kenacort oral paste 0.1%Applied thrice daily for 2 weeks | 27 patients | Male-16Female-11,31-60 yrsStudy group-44.46 yrsControl group-45.9 yrs | Significant improvement in the erythema (p=0.002), but non-significant reduction in pain (p=0.697), and ulceration (p=0.291) | 7,15 days | No side effects with topical curcumin.Patient treated with triamcinalone acetonide developed superadded candida infection | Curcumin can be used as an alternative to steroid in the management of OLP | NRS, E&U scores |
| Chainani-Wu N et al., [11]Randomised, double-blind, placebo-controlled trial | Tab Placebo vs. Tab Curcuminoids | Group 1-PlaceboGroup 2-2000 mg curcuminoids/day for 7 weeks. Both Groups 60 mg prednisolone/day for first 1 week. | 33 patients | Male 10,Female 23,Group 1- 60.6 yrsGroup 2- 60.6 yrs | No significant difference in the change from baseline in VAS, NRS, erythema, ulceration, between the placebo and curcuminoids groups at weeks 1, 4, and 7. | Day 7,28,49 | In Curcuminoids Group-1 patient developed allergic reactionsPrednisolone Group-1 patient had skin reactions | Curcuminoids at this dose were well tolerated. | VAS, NRS, CSS, MOMI |
| Nosratzehi T et al., [41]Case control study | Curcumin vs. local. corticosteroids | Group 1- Mucoadhesive curcumin pasteGroup 2- 0.1% beta-methasone local steroid lotion. Nystatin suspension three times daily | 40 patientsGroup 1- 20Group 2- 20 | Female-26Male-14Group 1- 38.5±7.03Group 2- 41.9±11.22 | No significant difference between the groups treated with curcumin and local corticosteroids with p>0.05 | 1,2,4,8,12 weeks | No adverse effects | Reduction in burning sensation, severity and size of the lesion when treated with curcumin for oral lichen planus | VAS |
| Chainani-Wu N et al., [35]Randomised, double-blind, placebo-controlled clinical trial | Curcuminoids vs. placebo | Group 1- PlaceboGroup 2- 6000 mg of curcuminoids in 3 divided doses | 20 patients | Male-7Females-13Group 1- 56.2±11.7Group 2- 60.8±8.6 | Placebo group had no statistical significance with NRS p=0.99, erythema p=0.98, ulceration p=0.63 and MOMI p=0.95. Curcumin group had statistical significance with NRS p=0.0078, erythema p=0.0078, ulceration p=0.063 and MOMI p=0.0039 | 2 weeks | No adverse effects | Curcuminoids are well tolerated and may prove efficacious in controlling signs and symptoms of oral lichen planus | NRS, MOMI |
| Nigam N et al., [43]Comparative study | Tablet Turmeric,Topical application of turmeric and honey | Tab Turmix-Twice daily for 15 days, topical turmeric and honey paste 2-3 times daily | OSMF-60Leukoplakia-10OLP-30 | Gender, age not mentioned | Significant decrease in VAS. 6.91 to 3.98Z value-8.642p-value <0.001 | 15 days | No side effects | Turmeric and honey showed positive results in reducing burning sensation in PMDs | VAS |
| Kia SJ et al., [45]Pilot clinical trial intervention study | Curcumin capsules | Curcumin capsules 80 mg, one capsule every day after breakfast for 4 weeks | 10 patients | 48.4±10.13 yearsFemale-9, Male-1 | Statistically significant differences in pain intensity (p=0.043) and clinical appearance of oral lesions (p=0.001) | 0,1,2,4 weeks | Few side effects | Oral curcumin nanomicelle formulation is an alternative treatment for OLP | VAS, Tgpm |
| Biswas S et al., [40]Randomised clinical trial | Curcumin oral paste | 1% curcumin oral paste once daily for 60 days | 10 patients | Male-3, Female-7, 26-61 years | Burning sensation, Appearance score-90% complete remission. | 60 days | No side effects | Curcumin can be suggested for treatment of OLP. | VAS, Tgpm |
OLP: Oral lichen planus; OSMF: Oral submucous fibrosis; PMDs: Potentially malignant disorders; CSS: Change in symptom scale; E&U scores-Erythema & ulcer scores
Characteristics of Participant
The number of participants who took part in intervention were twenty to seventy-five in number. Overall, 325 OLP patients were included in this systematic review, female participants were 194 and male patients were 91 and 40 cases where gender was not specified.
Among the 12 studies, five examined Atrophic/Erosive OLP [36-39,41], seven studies didn’t mention their clinical types [11,35,40,42-45].
Characteristics of Intervention
There were 12 selected studies that had different formulations of curcumin. Three used curcumin tablets [11,35,36], three used ointments [39,42,44], one used gel [38], three used oral paste [37,40,41] among which one used muco adhesive paste [41], one used both topical and oral turmeric [44], curcumin capsule [45]. Five studies prescribed the intervention once daily [11,35,36,40,45], three trials twice daily [42-44], four trials prescribed three times daily [37,39,41] in one study Group 2-thrice daily, Group 3-six times daily [38]. One study had mentioned to apply oral paste after meals [41], after breakfast [45].
Clinical Parameters
Parameters assessed clinically were Visual Analogue Scale (VAS) in 9 studies [11,36,37,40-45] Numeric Rating scale (NRS) in 5 studies [11,35,38,39,42]. Thongprasom in 6 studies [36,37,40,42,44,45] Modified Oral Mucositis Index (MOMI) in 4 studies [11,35,38,39].
Grading of severity index and pain index in one study [41], change in symptom scale in one study [11].
Risk of Bias
Among the twelve clinical trials, the estimated risk of bias was moderate in 5 studies [41-45] and low in 7 studies [11,35-40].
Study Outcome
Pain by VAS
Two studies showed VAS score were reduced in placebo and oral curcuminoids groups at first and second visits with statistically significant difference but not statistically significant when observed between 2 groups [11,35]. In two studies, differences between the two groups treated with curcumin and triamcinolone acetonide 0.1% were not significant, pain index decreased during the follow-up sessions [37,41]. Five studies [40,42-45] showed significant improvement in reducing pain in curcumin/turmeric group.
Clinical improvement by Thongprasom (Tgpm) et al., score
Amirchaghmaghi M et al., showed complete remission of atrophic/erosive lesions in 75% of curcumin group compared to 62.5% of control group patients. There was no statistically significant difference between the two studied groups with reduction in both groups at first and second visits [36]. Kia SJ et al., showed the mean Thongprasom score to be 3.5±1.08 at baseline, which decreased to 3.4±1.17, 3.0±1.24, and 2.5±1.17 at the second, third, and fourth visits respectively [45]. Biswas S et al., showed 90% complete remission [40].
NRS and MOMI score
Chainani-Wu N et al., reported that curcuminoids group showed a greater reduction in clinical signs and symptoms when compared to the placebo group, measured by percentage change in erythema (p=0.05) and total MOMI score (p=0.03), and proportion showing improvement in NRS (0.8 vs. 0.3, p=0.02) and total MOMI score (0.9 vs. 0.5, p=0.05) [35].
Size of the lesion
Nosratzehi T et al., found that the lesion size and pain severities exhibited no significant differences between the curcumin and local corticosteroids (p>0.05) [41].
Discussion
Oral Lichen Planus is a chronic mucocutaneous, autoimmune condition, which is a potentially malignant disorder that can transform into oral cancer [44-46]. Erythematous or erosive areas has impact on quality of life and are a source of morbidity [47,48]. There is no known and effective treatment available till now for oral lichen planus. Topical steroids are the mainstay treatment for OLP due to their chronicity and recalcitrant nature. Despite its therapeutic action, they have adverse effects due to long-term use that reduces the efficacy of treatment [8].
The aim of this systematic review was to assess the effect of curcumin on pain reduction and clinical improvement in symptomatic OLP. This systematic review has included 7 Randomised clinical trials, 3 comparative studies and 2 pilot studies.
Data obtained revealed heterogeneity in age of participants, study design, duration, characteristics of participants, intervention, clinical parameters, ninety-one were males, one hundred and ninety-four were female patients with erosive/atrophic OLP.
Duration of treatment varied from 2 weeks to 4 months. There was difference in control agents used. In 3 studies placebo tablets were control [11,36,38], in one study, tulsi in glycerin base [42], in one only glycerin base [43], 4 trials used triamcinolone acetonide [11,38,39,41], 3 studies had no control agents [39,43,44].
Outcome studied were VAS score, NRS Score, Thongprasom score (clinical improvement), Corozzo & gondolfo for treatment response and size of lesion was also measured.
In Amirchaghmaghi M et al., and Chainani-Wu N et al., study there was no significant difference in pain reduction by VAS score between oral curcumin (with dosage of 2000 mg/day) and placebo [11,36]. Chainani-Wu N et al., observed that with increase in dosage of oral curcumin 6000 mg and longer duration of administration, there was significant difference achieved when compared to placebo group [35]. In the study by Kia SJ et al., Thomas AE et al., Keshari D et al., Nozaretzehi T et al., there was no significant difference between topical curcumin and topical corticosteroids. These studies showed intragroup significance from baseline comparison to follow-up visits [37-39,41].
Kia SJ et al., revealed that there was comparable effect in pain reduction and appearance score in both triamcinolone and curcumin with no statistical significant difference between two groups. Effects were similar to those of topical corticosteroids, and thus, it can be a suitable alternative to corticosteroids [37]. Nozaretzehi T et al., stated that curcumin was effective in the treatment of oral lichen planus lesions and resulted in decrease in lesion sizes, pain and burning sensation without any complications [41].
Singh V et al., found that curcumin was effective in reducing pain and white lesions as curcumin used in the study was crude extract mixed with glycerine at a ratio of 75:25 [42]. Keshari D et al., examined significant improvement in the erythema, but non-significant reduction in pain and ulceration in the topical curcumin group as compared to the triamcinolone acetonide group [39].
Thomas AE et al., in her study assessed increased reduction of burning sensation measured by NRS, observed in triamcinolone acetonide group, followed by curcumin 6 times daily application [38]. Curcumin oral gel, applied 3 times a day, was insignificant compared to TA. Application of curcumin oral gel six times daily was considered equally effective compared to triamcinolone acetonide. There was comparable effect in pain reduction score in both triamcinolone and curcumin groups with no statistical significant difference between two groups. Effects were similar to those of topical corticosteroids, and thus, it can be a suitable alternative to corticosteroids.
Of the included studies, three trials reported adverse effects of curcumin such as burning sensation, itching, mild swelling and xerostomia, which disappeared spontaneously [37]. In another study, curcuminoids group patient developed allergic reactions [9], diarrhoea at higher doses [35]. Kia SJ et al., used oral curcumin nanomicelles and showed pain reduction in 50% and clinical appearance of oral lesions in 80% of the patients [45]. Nigam N et al., showed decrease in the mean VAS score from 6.91-3.98 [43], In a study by Biswas S et al., there was reduction in burning sensation and 90% showed short complete remission [40].
The clinical trials [37-39,41] that compared curcumin with corticosteroids agreed that curcumin is a safe effective alternative modality for management of OLP and with increase in frequency of applications, curcumin effects were comparable to corticosteroids [8], 3 clinical trials [11,35,36] compared with placebo reported that curcumin was effective with increased dose and was also safe and well tolerated. One comparative and pilot study [42,43] revealed curcumin to be effective in management of OLP with increased concentration. Three studies with no controls were effective in reducing pain, burning sensation and clinical appearance, out of which, one study used oral curcumin nanomicelle formulation as an alternative treatment for OLP.
From the above 12 clinical trials, curcumin can be used as a maintenance or an adjunct drug after initial treatment with corticosteroids. Curcuminoids are well tolerated and may prove efficacious in controlling signs and symptoms of oral lichen planus but still there is insufficient evidence to support the effectiveness of curcumin over placebo or steroids in OLP management. Though few clinical trials are positive and promising, applicability of topical curcumin in OLP treatment remains moderate. Standardised, novel curcumin formulations are recommended which increases its efficacy, bioavailability and improved effectiveness in patients which can then be compared to those of corticosteroids.
Limitation(s)
Heterogeneity in studies, few RCTs, small sample size, heterogeneity in follow-ups, curcumin formulations, methodological quality control, outcome measures due to which meta-analysis couldn’t be done.
Conclusion(s)
This systematic review highlights the significant advantages of curcumin in alleviating signs and symptoms of oral lichen planus owing to its many beneficial properties. The positive results in a few studies proves its effectiveness, however a few studies provided moderate evidence in treating oral lichen planus. From the above 12 articles, it has been concluded that it is safe, well tolerated, can be used with least side effects but can be done so with an increased sample size with long follow-up periods.