Thyroid gland disorders are very common in both Iodine sufficient and Iodine deficient areas. Kerala is no exception. It has a vast coastline with sea food rich in Iodine. Salt consumed regularly by Keralites is Iodised. It also has a long shore with radioactive minerals, thus subjecting the population to terrestrial radioactivity. All these accumulate to increase the incidence of thyroid disorders in Kerala [1,2].
Among all the thyroid disorders seen in Keralites, most common is the nodular colloid goitre. Most of these lesions show, either focally or diffusely, features seen in thyroid malignancies like, nuclear enlargement, clearing, grooving, cytoplasmic eosinophilia etc. This poses difficulty in differentiating non-neoplastic from neoplastic lesions.
As stated by Lanier LL et al., CD56- a Neural Cell Adhesion Molecule (NCAM) is expressed on essentially all human NK cells and on a subset of T lymphocytes and IL-2-activated thyroid follicular cells and also on normal thyroid follicular cells [3]. It is observed as membrane positivity as put forth by Zeromski J et al., in their study using this marker [4]. This adhesion molecule is said to play a role in thyroid follicular cell differentiation [4]. Reduced expression or loss of CD56 is implicated in tumour progression in patients with thyroid cancer, mainly PTC as stated by previous studies [5-7].
The p63 is a p53-homologue nuclear transcription factor and expressed by the basal layer of squamous epithelium, myoepithelial cells in the breast and the prostate and the transitional epithelium of the bladder. The p63 is not normally expressed in thyroid epithelial cells. Therefore, abnormal p63 expression might constitute an alternative mechanism to circumvent p53 activity in thyroid carcinogenesis and tumour progression [8].
This study was conducted to assess the expression of CD56 and p63 in the various thyroid lesions. As per the search, very few such studies have been performed in Kerala. The plan is to expand the study to evaluate the utility of a combination of CD56 and p63 as a diagnostic tool for PTC in cases of diagnostic dilemma.
Materials and Methods
The present study was conducted prospectively and included all thyroidectomies received in the Department of Pathology, Govt. TD Medical College, Alappuzha, Kerala, India. The study duration was from January 2016 to June 2017. Institutional ethical clearance was obtained from institution (IEC No. is EC 103/2015- ECR/122/Inst/KL/2013).
Most common lesion identified on H&E stained sections of these specimens was colloid goitre with follicles showing flattened lining and abundant colloid. Identifying positive reaction to IHC was difficult in the flattened follicular lining epithelial cells in colloid nodules. Also, the abundant colloid was found to take up the stain giving an unwanted background staining. Moreover, many of these cases showed a component of thyroiditis or some other lesions. Hence, pure colloid goitres were excluded from the study and 100 consecutive non-colloid goitrous lesions or colloid goitres associated with other lesions, obtained over the study period were evaluated for IHC markers, CD56 and p63.
IHC was performed using Polyexcel HRP/DAB system of PathnSitu Biotechnology Pvt., Ltd., Paraffin embedded, 4 micrometre thick tissue sections were prepared on Poly L Lysine coated slides, and deparaffinised. Antigen retrieval was by Tris Buffer EDTA in a Multi-Epitope Retrieval System at medium heat for 15 minutes. Internal peroxidase was blocked using H2O2 in methanol. Primary Antibody used for CD56 was PathnSitu Mouse Monoclonal antibody, Clone123C3 and for p63 was PathnSitu Mouse Monoclonal antibody, Clone 4A4. All washes were with phosphate buffer. After applying HRP, DAB chromogen was used to bring out the reaction. Normal thyroid tissue in the specimen acted as positive control for CD56 and normal skin was taken as positive control for p63. Negative controls were created by omission of primary antibody and replacement with phosphate buffer saline. A positive membranous staining without a cytoplasmic staining in 10% or more neoplastic cells was considered as positive staining for CD56 and any nuclear staining was counted as positive for p63.
Statistical Analysis
The lesions were tabulated in a Microsoft Excel sheet and percentage of lesions showing positive and negative reactions to each marker were calculated and compared with other similar studies.
Results
A total of 100 thyroidectomy cases were included in the study. Lymphocytic thyroiditis was the most common lesion comprising 39% (39 out of 100 cases) of all thyroid lesions. PTC was the most common malignancy comprising about 35% (35 out of total 100 cases) of all thyroid lesions in the present study. Other lesions which were studied, included, Hashimoto thyroiditis (11 cases) and cellular nodule (8 cases) as non-neoplastic conditions. There were 3 cases of benign neoplasms i.e., follicular adenoma. Apart from PTC, other malignancies studied, included two cases of Hurthle cell carcinoma, and one case each of Medullary carcinoma and Follicular carcinoma.
In the present study, it was observed that 53 out of 58 non-neoplastic lesions i.e., 91.4% and 4 out of 42 neoplasms i.e., 9.5% (both benign and malignant), showed positive membrane staining with CD56.
A total of 38 out of 39 (97.4%) lymphocytic thyroiditis, 10/11 (90.9%) Hashimoto thyroiditis, and 5/8 (62.5%) cellular nodules were positive for CD56. One case each of lymphocytic thyroiditis, Hashimoto thyroiditis and cellular nodule was negative for CD56. Two cases of cellular/hyperplastic nodules were inconclusive.
Benign neoplasm, follicular adenoma, showed 66.7% (2 out of the 3 cases studied) positivity for CD56. One case out of the 35 (2.85%) cases of PTC studied and the only case of Medullary carcinoma showed CD56 positivity. A total of 33/35 cases (94.2%) of PTC, 2/2 cases of Hurthle cell carcinoma and the single case of follicular carcinoma in the present study were negative for CD56. One case of PTC was inconclusive for analysis.
The staining pattern of p63 showed negative nuclear staining reaction in most of the lesions i.e., 81 out of 100 cases, irrespective of the nature of the lesions, while only 18 cases, including both non-neoplastic and neoplastic lesions, showed nuclear positivity. These included 3/39 cases (7.7%) of lymphocytic thyroiditis and 2/11 cases (18.2%) of Hashimoto thyroiditis and 13/35 cases of PTC (37.1%) which showed p63 positivity. A total of 7/8 cases (87.5%) of cellular nodules studied were negative. One case of cellular nodule was inconclusive for assessment. All the other benign and malignant lesions were negative for p63 staining reaction.
The [Table/Fig-1] shows the number of cases showing positive and negative staining reactions for CD56 and p63 in various thyroid lesions.
shows the numbers of various lesions which showed positive, negative and inconclusive staining for CD56 and p63.
| Lesions (numbers) | Immuno-staining |
|---|
| CD56 | p63 |
|---|
| Positive | Negative | Inconclusive | Positive | Negative | Inconclusive |
|---|
| Papillary thyroid carcinoma (35) | 1 | 33 | 1 | 13 | 22 | 0 |
| Hashimoto thyroiditis (11) | 10 | 1 | 0 | 2 | 9 | 0 |
| Lymphocytic thyroiditis (39) | 38 | 1 | 0 | 3 | 36 | 0 |
| Follicular adenoma (3) | 2 | 1 | 0 | 0 | 3 | 0 |
| Cellular nodule (8) | 5 | 1 | 2 | 0 | 7 | 1 |
| Hurthle cell carcinoma (2) | 0 | 2 | 0 | 0 | 2 | 0 |
| Medullary carcinoma (1) | 1 | 0 | 0 | 0 | 1 | 0 |
| Follicular carcinoma (1) | 0 | 1 | 0 | 0 | 1 | 0 |
| Total (100) | 57 | 40 | 3 | 18 | 81 | 1 |
Discussion
Thyroidectomy specimens constitute a significant proportion of histopathology specimens in any institution. Most surgeries are performed for goitrous thyroid enlargements due to disordered thyroid function, thus, making nodular colloid goitre, as the most common thyroid gland disorder all over the world. Hyperplastic/cellular nodules are non-neoplastic lesions commonly seen against a background of colloid goitre, presumably because of an intrinsic genetic abnormality in some follicular cells, giving them a growth potential which leads to polyclonal and monoclonal nodule formation [9].
Next most common category of diseases affecting thyroid gland in Keralites is immune mediated diseases like lymphocytic/Hashimoto thyroiditis which involves breakdown of self-tolerance to thyroid autoantigens leading to thyroid cell death [9]. Neoplasms of the thyroid gland are also very common, most common being PTC. The diagnostic features of these lesions are defined; however, sometimes a diagnostic difficulty is encountered. Expression of many IHC markers on thyroid gland has been studied and their usefulness in diagnosing malignancies were evaluated. Two such markers are CD56 and p63 [10-16].
CD56 is a neural cell adhesion molecule and the prototypic marker of NK cells (80-90%) [3]. It is also present on subsets of CD4+ and CD8+ T cells which mediate the development of lymphocytic/Hashimoto thyroiditis [3]. Studies have shown that normal thyroid follicular epithelium also expresses CD56 where it is assumed to play a role in thyroid follicular cell differentiation [4]. The p63, a member of the tumour suppressor gene family, is the homologous nuclear transcription factor of p53. As the thyroid follicular cells are derived from endodermal progenitor cells, p63 is normally not expressed in thyroid epithelial cells. Hence, p63 expression might constitute pathogenic mechanism in thyroid carcinogenesis [8].
The [Table/Fig-2,3,4,5,6 and 7] show the staining characteristics of various thyroid lesions with CD56 and p63.
Shows cellular nodule: a&b) H&E at X100 and X400 magnification; c) shows membranous positivity for CD56 (X400 magnification); d) shows negative p63 expression (X400 magnification).
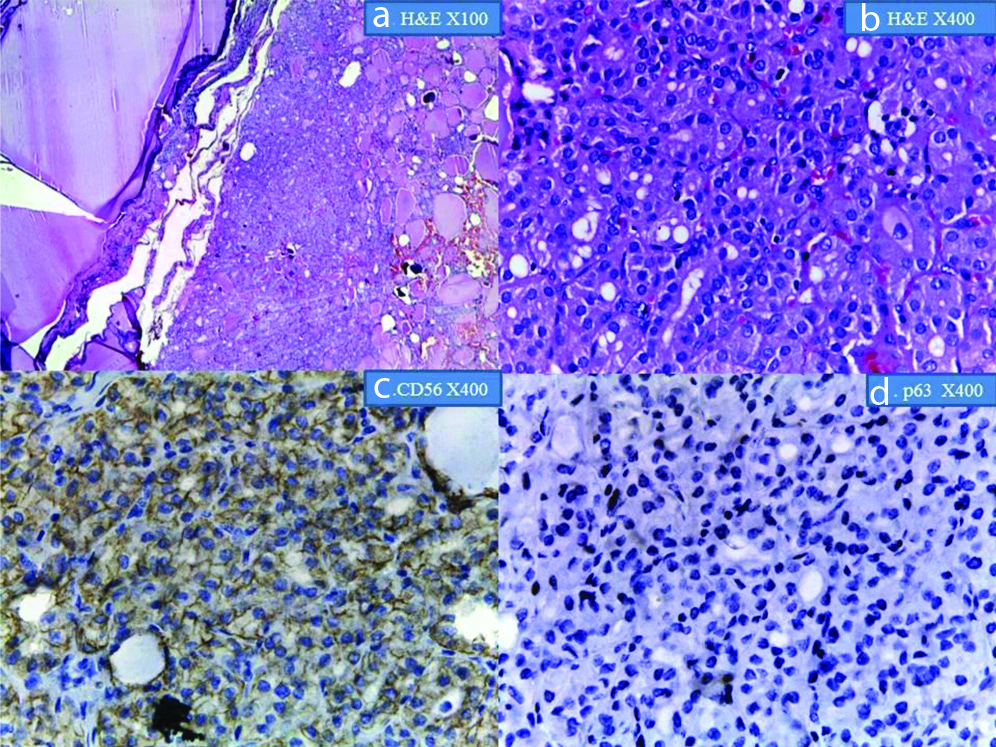
Shows lymphocytic thyroiditis: a&b) H&E at x40 and X400 magnification; c) shows membrane positivity for CD56 at x400 magnification; d) shows negative p63 reaction at X100 magnification.
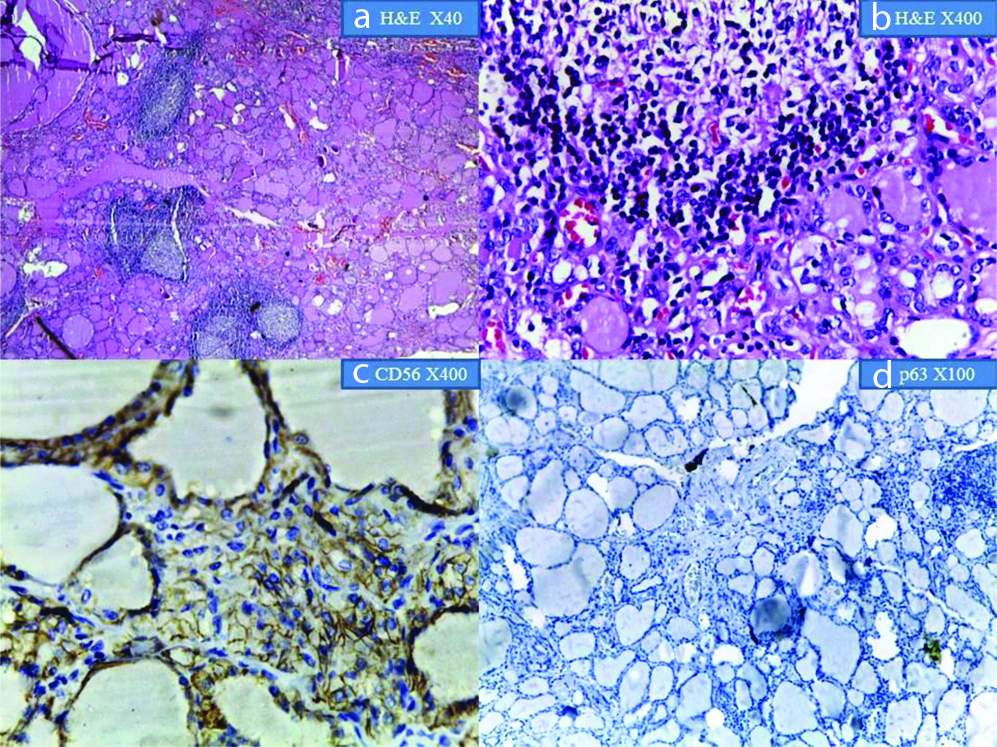
Shows Hashimoto thyroiditis: a&b) at X100 and X400 magnification; c) shows CD56 positive follicular cells (X400 magnification); d) shows p63 expression in follicular cells at X100 magnification.
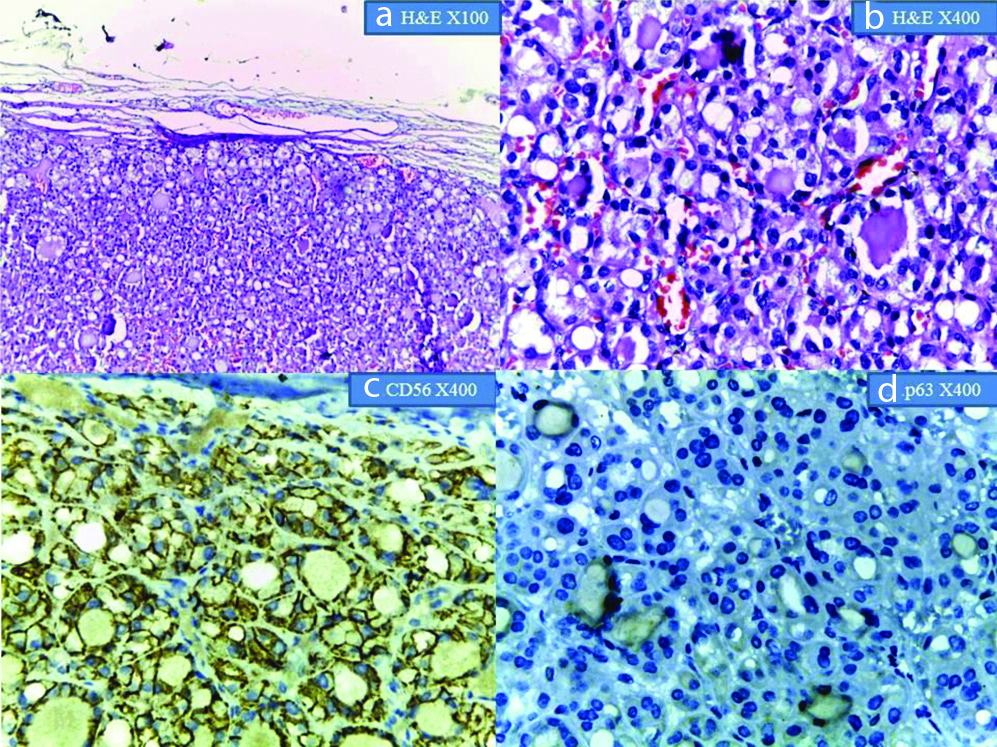
Shows Follicular Adenoma: a&b) H&E at X100 and X400 magnification; c&d) show CD56 positivity and p63 negativity at X400 magnification, respectively.
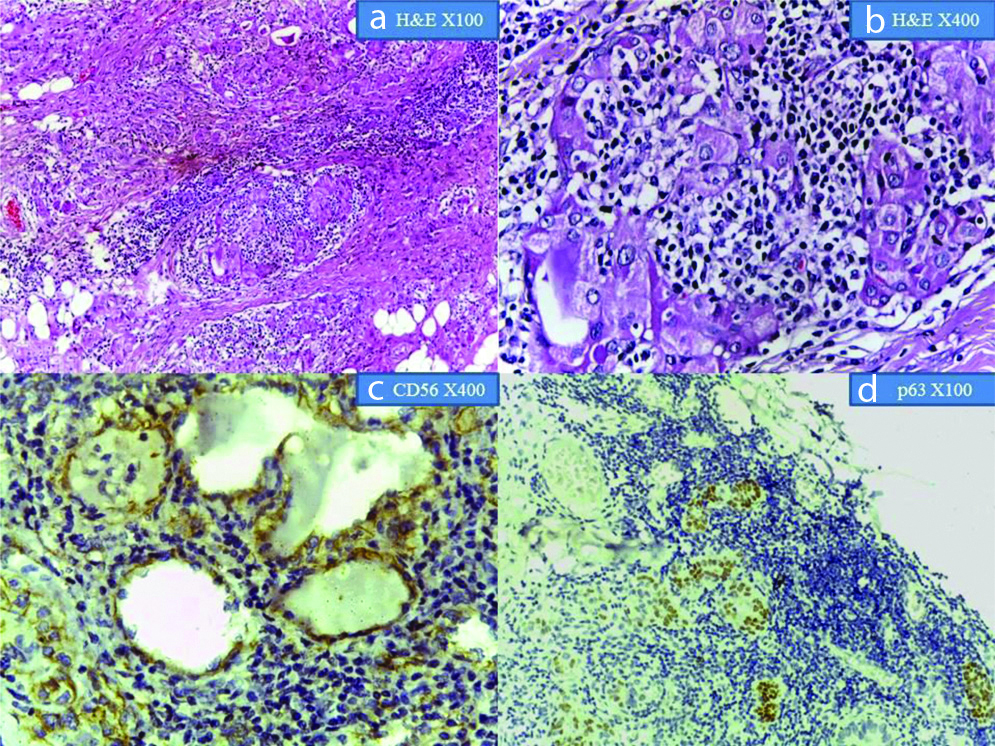
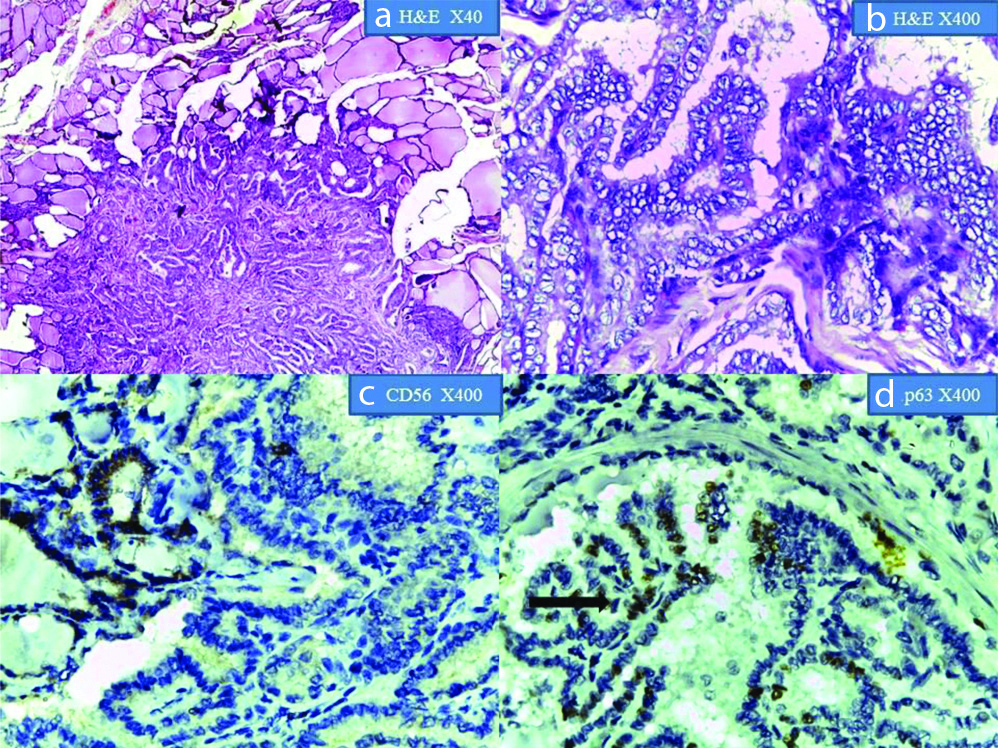
Show Papillary Thyroid Carcinoma in H&E at x40 and X400 magnification; (c) CD56 showing membrane positivity in the normal thyroid follicle cells. Adjacent focus of PTC shows negative expression (X400 magnification); (d) p63 showing nuclear positivity in PTC(arrow). Adjacent normal tissue shows negative expression (X400 magnification).
Follicular Carcinoma: a&b) H&E at x40 and X100 magnification respectively, showing capsular invasion; c&d) showing absent expression for CD56 and p63 respectively at X400 magnification.
Ozolins A et al., noted that CD56 expression was significantly increased in Follicular adenoma compared to its expression in surrounding normal thyroid tissue [10]. However, the expression was markedly reduced in PTC. In the present study, while, CD56 was expressed by most follicular adenomas and was negative in most PTCs, the present authors could not find a difference in the staining expression between normal and the adenomatous area, possibly due to differences in the fixation and antigen retrieval processes.
El Demellawy D et al., showed 100% positivity for CD56 staining in all non-neoplastic lesions included in their study [11]. A complete absence of CD56 staining was observed in all their cases of PTC as also in the metastatic deposits from these lesions. Present study did not show a 100% positivity or negativity for CD56 staining reaction. The number of non-neoplastic lesions in their study was much less compared to the present study while the number of neoplasms was considerably more. Study population differences, effect of environment not only on gland but on the disease process and also on the technique of processing the tissue, might be the cause for this difference. Shin MK et al., also observed positive CD56 staining reaction in most of their non-neoplastic and benign neoplastic lesions, but was found to be negative in most of the PTC studied [12]. In spite of the fact that the present study had fewer neoplastic lesions compared to theirs’, the present findings correlated considerably with their study. Jeong JY et al., studied only follicular patterned lesion and observed an increased CD56 expression in non-neoplastic and benign neoplastic lesions while the expression was decreased in PTC and Follicular carcinoma [13]. Follicular carcinoma and Hurthle cell carcinoma were negative for CD56 in the present study also. Their study did not include thyroiditis, hence a comparative analysis in this category of lesions could not be done. Etem H et al., also studied only follicular neoplasms and PTC and found an absent CD56 staining in 35% of both follicular neoplasms and papillary carcinomas, possibly due to small sample size [14]. Differences in study population might be the reason for the differences between our studies. Ayse BC et al., who categorised all lesions with nuclear features similar to PTC into one category, suggested a semi-quantification of the CD56 expression, based on cytoplasmic and membrane positivity [15]. They found that CD56 positivity and E-cadherin positivity was significantly increased in follicular lesions, whereas, CD56 negativity and E-cadherin loss was significantly increased in PTC. The present authors found a positive reaction pattern with CD56 in follicular lesions and a reduced or absent expression in PTC correlating well with their study. Mozhgan M et al., included all non-neoplastic and neoplastic lesions of thyroid as a common group and differentiated PTC from these lesions on the basis of CD56 staining reaction [16]. Findings of present study with regard to the non-PTC lesions differed from the above mentioned study possibly due to sample size and study population [Table/Fig-8] [11-16].
Positive CD56 Expression in various thyroid lesions- a comparison with other studies [11-16].
| StudiesLesions | El Demellawy D et al., [11] | Shin MK et al., [12] | Jeong JY et al., [13] | Etem H et al., [14] | Ayse BC et al., [15] | Mozhgan M et al., [16] | Current study |
|---|
| Hashimoto thyroiditis | 6/6 (100%) | 4/5 (80%) | - | | | 70/73 (95.8%) | 10/11 (91%) |
| Lymphocytic thyroiditis | 19/19 (100%) | - | - | | 55/60 (91.7%) | 38/39 (97.4%) |
| Cellular nodule | 25/25 (100%) | 7/12 (58.33%) | 31/40 (77.5%) | | 5/8 (62.5%) |
| Follicular adenoma | - | 5/9 (55.5%) | 27/40 (67.5%) | 14/40 (35%) | 2/3 (66.7%) |
| Follicular carcinoma | - | - | 14/40 (35%) | 0/1 (0%) |
| Hurthle cell carcinoma | - | - | - | | | 0/2 (0%) |
| PTC | 0/72 (0%) | 4/80 (5%) | 69/129 (53.5%) | 14/40 (35%) | 9/101 (8.9%) | 1/73 (1.4%) | 1/35 (2.9%) |
Three of all the 100 cases in the present study, which included one case of PTC, and two cases of cellular nodules, did not show expected staining pattern with CD56 and were considered inconclusive. It was seen that even the internal control, the adjacent normal thyroid tissue, had not stained positive with CD56 or p63. This was in spite of a repeat IHC. This may be due to technical problems like over or under-fixation of the specimen or excessive dehydration or pH variation, improper antigen retrieval etc.
In the present study, nuclear positivity for p63 was seen in 13 out of 35 cases of PTC, 2 out of 11 cases of Hashimoto and 3 out of 39 cases of lymphocytic thyroiditis. Burstein DE et al., analysed the expression of p63 expression in PTC, Hashimoto thyroiditis and solid cell nest often seen associated with almost all cases of Hashimoto thyroiditis and many PTCs [8]. They found these cells p63 positive. They also observed p63 nuclear positivity in 7 of 27 cases of PTC in their study. They suggested a causal relationship of Hashimoto thyroiditis to PTC based on the presence of these solid cells rests in both lesions. This may explain the positivity in the present study also. Moreover, RET proto oncogene is usually expressed in cells of neural crest origin. Rearrangement of RET gene, known as RET/PTC gene which is frequently seen to be associated with PTC, is also expressed in some of the benign conditions such as Hashimoto thyroiditis, hyperplastic nodules, follicular adenoma etc., [17].
Unger P et al., observed a negative p63 expression in normal thyroid tissue, nodular goitres, and oncocytic follicular adenomas [18]. A weak positivity was seen in follicular adenomas and p63-positive foci were seen in 78.8% of cases of Hashimoto thyroiditis so also in 27 out of 33 cases of PTC in their study. None of the follicular neoplasms (including 3 cases of follicular adenoma, 1 case of follicular carcinoma and 2 cases of Hurthle cell carcinoma) in the present study, showed positive expression for p63. This is in concordance with the previous studies [Table/Fig-9] [13,14,19,20].
Positive p63 Expression in various thyroid lesion- a comparison with other studies [13,14,19,20].
| StudiesLesions | Jeong JY et al., [13] | Etem H et al., [14] | El Demellawy D et al., [20] | Kim YW et al., [19] | Current study |
|---|
| PTC | 19/129 (14.7%) | 12/40 (30%) | 70% | 5/40 (12.5%) | 13/35 (37.1%) |
| Hashimoto thyroiditis | - | - | 0/65 (0) | - | 2/11 (18.2%) |
| Lymphocytic thyroiditis | - | - | | - | 3/39 (7.2%) |
| Follicular adenoma | 1/40 (2.5%) | 2/35 (5.7%0 | | - | 0/3 |
| Cellular nodule | 0/40 (0) | - | | - | 0/8 |
| Follicular carcinoma | 0/40(0) | 1/3 (33.3%) | - | 0/40 | 0/8 |
| Hurthle cell carcinoma | - | - | - | - | 0/8 |
| Medullary carcinoma | - | - | - | 0/10 | 0/1 |
| Others | - | 0/2 (0) | | 6/16 | - |
Most of the studies mentioned have assessed the staining reaction of p63, predominantly, in neoplastic lesions. There is limited data on its expression in non-neoplastic thyroid conditions.
One of the case in the present study for cellular nodules showed diffuse cytoplasmic and nuclear staining for p63, i.e., inconclusive for p63 reaction, despite a repeat IHC. This was in addition to it showing an inconclusive CD56 reaction pattern as already discussed. Possibility of a technical error of improper fixation or processing or pH variation might have led to this result.
Limitation(s)
The sample size of cases fulfilling the inclusion criteria was small and needs to include more number of lesions, both benign and malignant.
Conclusion(s)
In the present study, CD56 was found positive in only 5.1% of all thyroid gland malignancies and 90.1% of all non-neoplastic and benign neoplastic lesions of the thyroid gland. p63 was negative in most of the thyroid gland lesions irrespective of the nature of the lesions. The study needs to be repeated on more number of cases to calculate a statistical significance. Also, more number of malignancies need to be studied to assess the usefulness of combination of CD56 and p63 in diagnosing PTC in cases of ambiguity.