Type 2 DM is the most common type of DM. The prevalence of this disease can be justified by obesity over the past decade [1]. By 2017, the DM prevalence was reported as nearly 8.8% (20 to 79 year) worldwide, and this percentage estimated to be increased to 9.9% in 2045 [2]. Approximately, 4.0 (3.2-5.0) million people aged between 20 and 79 years were estimated to die due to DM in 2017 [2,3]. Based on international diabetes federation reports, global health expenditures for diabetes prevention, treatment and its complications was estimated to be at least 376 billion US Dollar (USD) in 2010 and by 2030, this number will exceed 490 billion USD [4]. Despite existing therapies, only 50% to 70% of Type 2 DM patients achieve the therapeutic goals. Therefore, new therapeutic agents and treatment protocols should be considered [5,6].
Dopamine and dopaminergic signals control the Central Nervous System (CNS) activity [7]. Hence, it can be concluded that glucose metabolism can be precisely controlled by the CNS and the hypothalamic medioventral region [2]. The CNS regulates hepatic gluconeogenesis through the sympathetic nervous system; however, this pathway does not work in patients with DM and obesity whose signal response is impaired [8]. Additionally, hepatic gluconeogenesis, insulin resistance, and β cell dysfunction are observed in these patients. It has also been reported that factors affecting dopaminergic activity (for example, antipsychotics) cause side-effects such as metabolic disorders, weight gain, insulin resistance, and dysplasia [9-11].
Bromocriptine is a dopaminergic agonist which can affect serotonin function in CNS [3]. This agent is mainly used for the treatment of Parkinson’s disease, prolactinoma, acromegaly, infertility and galactorrhea over the last 25 years. Bromocriptine can also be used for the treatment of different insulin resistant and obesity associated metabolic disorders [9,10,12]. In addition, treatment with dopaminergic antagonists reduces the hypothalamus stimulation, increasing liver gluconeogenesis, lipid synthesis and insulin resistance [11,13]. Bromocriptine is also a Food and Drug Association (FDA)-approved agent as a choice for Type 2 DM management. Compared to the other glucose-lowering agents, bromocriptine is useful for the control of glucose level through affecting CNS and reduced glycogenesis [14-16]. Cycloset is a type of fast-release bromocriptine which reduces insulin resistance, hyperlipidemia, and hyperglycaemia in patients with Type 2 DM and obesity and is used as an anti-diabetic agent [17-19].
Long-acting cabergoline in low doses show less side effects and patients also show more compliance towards it [20-23].
However, there are few studies on the effects of cabergoline regarding the blood glucose control and insulin resistance. Regarding the above-mentioned issues, this study aimed to investigate the effects of cabergoline on blood glucose control in patients with Type 2 DM.
Materials and Methods
Forty-four patients with Type 2 DM on daily 2 grams of metformin, which could not achieve glycaemic goals, referred to outpatient clinics of Imam Reza hospital at Tabriz University of Medical sciences, were enrolled in this double-blind randomised controlled from September 2018 to September 2019. This study was approved by the Ethics Committee of Tabriz University of medical sciences (ethical code: IR.TBZMED.REC.1396.1291), and registered in Iranian Registry of Clinical Trials (IRCT) (code: IRCT2010031400356N9). Written informed consent was obtained from all individuals before any intervention.
For sample size evaluation, the mean and magnitude of the HbA1c as the major variable were estimated based on the Taghavi SM et al., study [1]. Considering the 95% confidence and 80 test power and taking into account the 5% change in this main variable, the minimum volume of the sample size was estimated as 19 in each group. Considering the dropouts, 22 patients were estimated for each group. The subjects were then randomly assigned into two case and control groups by an online application (www.stat.ubc.ca/Nrollin/stats/ssize/b0.html). The intervention group received 0.25 mg weekly Cabergoline tablets (Caberlin Iran Hormone Company) for the first 2 weeks and 0.5 mg per week for the next 12 weeks. Control group received placebo tablets similar to Cabergoline tablets in shape and packages [Table/Fig-1].
CONSORT flow chart of subjects in the study.
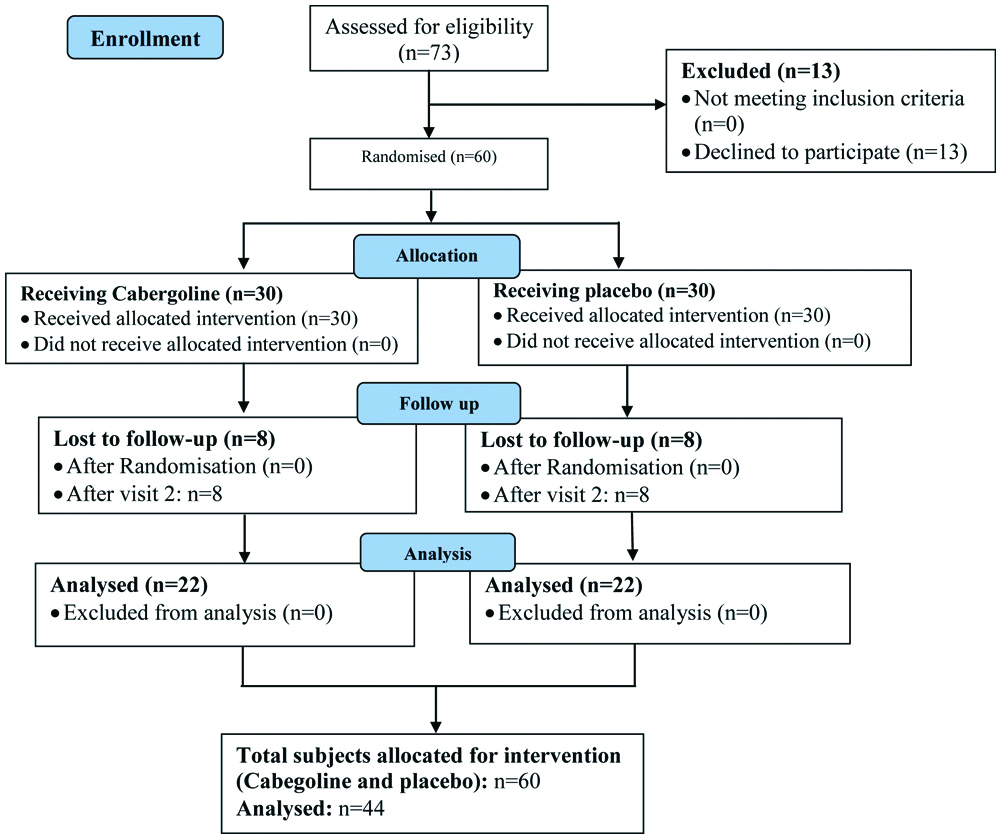
Inclusion Criteria
Patients with Type 2 DM receiving metformin at a dose of 2 grams per day for at least 3 months who could not achieve glycaemic control; age between 30 to 65 years; HbA1c ranged between 7.5-8%; and conscious consent to participate in the study were included.
Exclusion Criteria
Patients desiring to leave the study; pregnancy; cabergoline intolerance (any nausea, vomiting, stomach upset, constipation, dizziness, lightheadedness, or tiredness symptoms were considered as cabergoline intolerance); creatinine level >2 mg/dL during treatment; any clues of cardiovascular, kidney, liver, lung, thyroid and pituitary diseases; smoking; and taking any medications with effect on CNS or glycaemia other than metformin were excluded.
General physical examination; Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), height and weight measurement were also evaluated in all participants. Initial laboratory parameters including FBS, 2HPPG, HbA1c, T-Chol, TG, HDL were evaluated after an overnight fasting (at least 8 hours) using an autoanalyser instrument (BT3500, Italy). All clinical and laboratory studies repeated 3 months after initial visit. All patients received the routine treatment during study and advised not to change their diet and physical activity.
Statistical Analysis
Data were presented as mean±Standard Deviation (SD) and frequency or percent for quantitative and qualitative variables, respectively. The normality of the quantitative variables was also assessed by Kolmogorov-Smirnov (KS) test. Comparison of quantitative and qualitative variables (rank and nominal) was performed by paired t-test, independent t-test and chi-square, respectively. Independent t-test was used to compare changes in the main parameters or main variables between the intervention and control groups, and covariance analysis was used to adjust for confounding variables. Data analysis was performed by SPSS version 24.0 software. p<0.05 was considered as statistically significant.
Results
Forty-four patients (22 cases and 22 controls) were enrolled in this clinical trial study, of which 26 and 18 patients were female and male, respectively. The mean age of the patients in case and control groups was 50.54±7.8 and 53.45±6.9 year and the mean BMI of the mentioned groups was also calculated as 30.33±4.05 and 31.1±4.74, respectively. General characteristics of patients in both case and control groups are summarised in [Table/Fig-2].
General characteristics of participants.
| Parameter | Case | Control | p-value |
|---|
| Age (year) | 50.54±7.8 | 53.45±6.9 | 0.2 |
| Gender | Male | 5 | 13 | - |
| Female | 17 | 9 | - |
| Height | 161.76±6.9 | 162.50±7.6 | 0.74 |
| Weight | 79.18±12.4 | 82.29±12.33 | 0.4 |
| BMI (kg/m2) | Before | 30.33±4.05 | 31.1±4.74 | 0.58 |
| After | 30.36±4.05 | 30.49±4.34 | 0.92 |
| SBP (mmHg) | Before | 126.63±15.91 | 122.27±16.01 | 0.37 |
| After | 124.34±17.06 | 124.0±13.57 | 0.94 |
| DBP (mmHg) | Before | 79.77±20.54 | 79.31±8.8 | 0.39 |
| After | 75.68±9.16 | 79.63±6.5 | 0.89 |
Data are presented as mean±Standard division (SD) or number. p<0.05 was considered as statistically significant. BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure.
According to [Table/Fig-3], a significant decrease in HA1c was observed in case group after the intervention (8.04±0.35 versus 7.05±0.38, p<0.001). In the control group the mean HbA1c before and after the intervention was 8.14±0.38 and 7.59±0.92, respectively (p=0.013). The covariance analysis results showed a statistically significant reduction in the mean HbA1 levels in the case group compared to the control group (p=0.01).
Comparison of laboratory parameters between groups.
| Parameters | Case (N=22) | Control (N=22) |
|---|
| Before | After | p | Before | After | p | p* |
|---|
| HbA1c (%) | 8.04±0.35 | 7.05±0.38 | <0.0001 | 8.14±0.38 | 7.59±0.92 | 0.013 | 0.01 |
| FBS (mg/dL) | 145.09±31.46 | 139.95±34.38 | 0.607 | 173.86±31.88 | 149.36±36 | 0.021 | 0.36 |
| 2hpp (mg/dL) | 227.55±59.14 | 241.54±57.04 | 0.429 | 266.18±62.92 | 237.55±61.18 | 0.133 | 0.042 |
| TG (mg/dL) | 165.73±62.74 | 143.73±50.51 | 0.207 | 151.86±55.22 | 156.86±56.55 | 0.768 | 0.04 |
| Cholesterol (mg/dL) | 148.82±23.81 | 142.55±37.58 | 0.512 | 144.41±29.14 | 139.82±34.95 | 0.638 | 0.48 |
| LDL-c (mg/dL) | 74.25±16.02 | 68.89±25.13 | 0.403 | 77.77±34.17 | 71.64±31.73 | 0.54 | 0.41 |
| HDL-c (mg/dL) | 45.41±12.06 | 45.73±12.72 | 0.932 | 42.41±8.71 | 42.91±11.44 | 0.871 | 0.38 |
Data are presented as mean±standard division (SD). p<0.05 was considered as statistically significant. p: p-value before and after intervention among case and control groups. p*: p-value after intervention between case and control groups. FBS: Fasting blood glucose; 2HPP: 2 Hours post prandial; TG: Triglyceride; LDL: Low density lipoprotein-cholesterol; HDL-c: High density lipoprotein-cholesterol
The mean level of 2HPPG, however, showed a significant increase in case group after the intervention (227.55±59.14 versus 241.54±57.04). In contrast, 2HPP level was decreased in control group (266.18±62.92 versus 237.55±61.18) after intervention. The results of covariance analysis showed that the mean of 2HPPG in the case group was significantly higher compared to the control group (p=0.042).
Regarding the TG parameter, a decrease in TG level was observed in case group after the intervention (165.73±62.74 versus 143.73±50.51). However, in controls a slight increase was observed in TG levels after the intervention (151.86±55.22 versus 156.86±56.55). Covariance analysis also showed a significant reduction of TG parameter in case group compared to controls after the intervention (p=0.04). No statistical differences were observed in the remaining parameters between studied groups [Table/Fig-3].
Discussion
Considering the importance of the subject and the lack of sufficient international studies and the absence of a similar regional study, this study aimed to investigate the effect of cabergoline on glycaemic control in Type 2 DM patients receiving metformin with no satisfactory glycaemic control.
In this study, 44 patients were examined {case (n=22) and control (n=22) groups}. As shown in the results section, HbA1c and TG levels were significantly improved after the intervention in the case group compared to the controls, while FBS, T-Chol, LDL, and HDL levels were not significantly different between two groups after the intervention. On the other hand, a significant increase of 2HPP levels was observed in case groups compared to the controls. Cabergoline is a strong and long-acting dopamine agonist with higher effectiveness than that of bromocriptine in the treatment of hyperprolactinemia [20,24,25]. This agent is also considered as safe for the treatment of hyperprolactinemia [20,21]. In the present study, regarding the low dose and short-term administration of the drug, the side-effects were very minimal. However, any nausea, vomiting, stomach upset, constipation, dizziness, lightheadedness, or tiredness symptoms were monitored during the study. It has been reported that body weight will be significantly reduced in obese males with prolactinoma after six months of treatment with bromocriptine or cabergoline; however, this effect was not observed in female counterparts [25,26]. Another research group noted an improvement in insulin resistance and triglyceride levels after six months of treatment with bromocriptine or cabergoline; although, no significant changes were observed in BMI parameter in patients under study. It has also been shown that cabergoline use is associated with weight loss in patients with prolactinoma. Based on experimental evidence in animals and humans, it can be hypothesised that cabergoline may decrease food intake, body weight, and glucose tolerance.
Taghavi SM et al., showed that cabergoline reduces FBS levels in Type 2 DM patients with persistent hyperglycaemia [1]. Additionally, this research groups showed a significant decrease prolactin levels, after four months of treatment with cabergoline [1]. Moreover, Berinder K et al., in a study on 14 patients with prolactinoma reported a significant decrease in LDL-C levels after two months of cabergoline treatment [27]. Insulin sensitivity also tend to improve after six months of therapy; however, the insulin, Insulin-like Growth Factor-Binding Protein-1 (IGFBP-1) and total adiponectin levels were not significantly changed.
Although the present authors did not investigate prolactin levels in patients, similar results in terms of blood glucose reduction were observed. In this study, the increased levels of 2HPP in case group compared to the controls may be due to small sample size of the studied patients.
Some studies have also reported that cabergoline has a weight-reducing effect, but Venuti L and Zuppa A, and Gibson CD et al., were unable to observe this effect after 6 months of cabergoline treatment, similar to the present study [24,28]. In contrast Pala NA et al., in a study on 19 patients with prolactinoma showed a significant decrease in body weight after three and six months of cabergoline treatment with the dose of 0.5 mg orally/week compared to the controls [29]. Additionally, a significand decrease in BMI, waist circumference, waist-hip ratio and total body fat at six months of cabergoline treatment.
Limitation(s)
The evaluation of insulin levels and insulin sensitivity as well as IGFBP-1 levels could give better interpretation of the data.
Conclusion(s)
Cabergoline as a long-acting dopamine agonist may exert beneficial effects on HbA1C and TG over a 3 months period in Type 2 DM patients. However, the increased level of 2HPP after intervention is a controversial finding which should be further evaluated in future studies.
Funding: This study was supported by Endocrine Research Centre at Tabriz University of Medical Sciences, Iran [Grant No. 94/3-27/26].
Data are presented as mean±Standard division (SD) or number. p<0.05 was considered as statistically significant. BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure.
Data are presented as mean±standard division (SD). p<0.05 was considered as statistically significant. p: p-value before and after intervention among case and control groups. p*: p-value after intervention between case and control groups. FBS: Fasting blood glucose; 2HPP: 2 Hours post prandial; TG: Triglyceride; LDL: Low density lipoprotein-cholesterol; HDL-c: High density lipoprotein-cholesterol