Minoxidil a Youth Elixir for Eyebrow Hypotrichosis
Varsha Gajbhiye1, Yeshwant Lamture2
1 Associate Professor, Department of Pharmacology, J.N.M.C., Wardha, Maharashtra, India.
2 Professor, Department of Surgery, J.N.M.C., Wardha, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Yeshwant Lamture, J.N.M.C., Wardha-442005, Maharashtra, India.
E-mail: yash18671@gmail.com
Introduction
Eyebrows being prominent feature of the face contributes majorily for aesthetics. Eyebrow hypotrichosis means the reduction or absence of the eyebrow hair. This is associated with negative self-esteem. Minoxidil has been found to act at the level of hair follicle by direct stimulation of follicular epithelial growth, increase cutaneous blood flow, and stimulate resting hair follicles.
Aim
To study the potential role of Minoxidil for the treatment of eyebrow hypotrichosis.
Materials and Methods
It was a prospective interventional study comprising of 22 subjects aged 18 years or more having eyebrow hypotrichosis. These patients garded as Grade 1 or 2 hairloss on the Allergan Global Eyebrow Assessment (GEBA) scale. All the patients were advised to apply 1 mL of 2% minoxidil lotion to eyebrow twice a day for four months.
Comparison of the change in global photographic score (Subject’s satisfaction) before and after Minoxidil treatment was done, also comparison of the diameter and number of eyebrow hairs/cm2 areas between pre and post-treatment done. Paired t-test was used to compare diameter and number of eyebrow before and at the end of treatment.
Results
Before treatment with Minoxidil the average hair shaft diameter was 0.034±0.0057 mm and density was 17±5.03 hairs/cm2. After treatment this improved to 0.07±0.0045 mm and 30±7.03 hairs/cm2, respectively. The global photographic score was -2.1±0.76 before treatment that increased to -1.3±0.89 at four weeks and reached a peak of 2.3±0.55 at 16 weeks. The incidence of adverse events was 22.72% (5/22 subjects) which included mild itching and burning.
Conclusion
This study showed that 2% Minoxidil is a well-tolerated and effective treatment for eyebrow hypotrichosis.
Hair diameter, Madarosis, Photographic assessment
Introduction
Eyebrows plays a vital role in appearance and communication. Lack of growth or loss of eyebrow hairs is defined as Eyebrow hypotrichosis . It is usually idiopathic or related to an underlying disease. Movement of the eyebrows plays a dominant role in an individual’s ability to communicate effectively [1].
Although eyebrows are very important for facial appearance, eyebrow hypotrichosis is a neglected disorder hence obviously treatment options are few, other treatment options includes use of eyebrow pencils or tattoos; however, results of these options are not up to the mark. Safety of tattooing is not confirmed yet. The removal of cosmetic tattoos is costly and difficult. Surgical methods include flaps or hair transplantation [2].
The hair cycle of the eyebrow, consists of three phases: anagen, catagen, and telogen; similar to any other hair growth is present during anagen phase. Only 10 % of follicles are in growth phase in eye brows. Exogen is characterised as hair shedding phase followed by growth of new hair shaft in place of old ones. The anagen phase continues for 2 to 8 years on the scalp, and percentage of telogen hair follicles ranges from 10% to 15%. The anagen phase ranges from 30 to 35 days. The eyebrow hair at telogen ranges from 85% to 90%. The differences between scalp hair and eyebrows dictates the difference in development and application of treatments for these hairs [3].
It is known for years that Minoxidil stimulates hair growth. Minoxidil was found to limit the telogen phase converting resting hair follicles into the active ones [4]. Minoxidil may also cause prolongation of anagen and increase hair follicle size. Though not proven, minoxidil was found to act by opening potassium channels [5].
A study conducted by Greenland S and Robins JM, showed the efficacy bimatoprost over Minoxidil solution and placebo for the treatment of eyebrow hypotrichosis [6].
A study done by Worapunpong N and Tanglertsampan C, depicted good improvement in hair diameter and numbers in both minoxidil and placebo group at the end of 16 weeks [7]. This study does not show significant efficacy of minoxidil over placebo. Side effects were minor related to skin symptoms including temporarily mild itching and burning in the treatment areas.
Considering this confusing scenario in literature regarding efficacy and safety of Minoxidil especially for eye brow hair loss. Hence this study was carried out to know the safety and efficacy of Minoxidil in a treatment of eye brow hypotrichosis.
Materials and Methods
The present prospective interventional study was conducted for the duration of six months after getting approval of the Ethics Committee (Reference no DMIMS DU/IEC/2018-19/7149), on 22 patients (17 female and 5 male) selected from the dermatology outpatient department.
Calculation was done to obtain values of Zα, Z1-β, σ. Standard deviation was estimated, and Δ, the difference in effect of two interventions estimated. A p-value of <0.05 and a study with 80% power considered acceptable, after calculation got the following values: Zα as 1.96. Z1-β is 0.8416. For Δ [7],
The sample size for this study was 22:
N=2(Zα+Z1-β)2 σ2/Δ2
n=2(1.96+0.8416)2(0.72)2(0.61)2
=22
Inclusion Criteria
Eligible participants aged 18 years or more, with a Grade 1 or 2 on the GEBA scale were included. The scale is calibrated with 4 points used to grade fullness of the eyebrows (1=very sparse, 2=sparse, 3=full, and 4=very full) [8].
Exclusion Criteria
Thyroid diseases, pregnancy or breastfeeding, previous eyebrow tattoo, trauma or accident, history of eyebrow or hair medications in past six months, history of allergy to minoxidil or its ingredient, history of eyebrow surgery and paediatric age group were excluded from the study.
A 1 mL of 2% minoxidil lotion to eyebrow twice a day for four months was advised [7]. The primary endpoint for efficacy assessment was the change in global photographic scores from baseline. The secondary endpoints were the changes in hair shaft diameter and hair number at the end of 16 weeks. Side effects were also monitored. The subjects had a follow-up visit every four weeks.
Global photographic assessment or scale (Subject’s satisfaction) was performed by taking photographs by same clinician at baseline and during each visit. Its assessment was done using a 7-point scale [7]. Significantly worse (-3), Moderately worse (-2), Minimally worse (-1), No change (0), Minimally improved (+1), moderately improved (+2), and Significantly improved (+3).
Hair count and diameter were measured within 1-cm diameter circular areas using Folliscope, eyebrow density was evaluated by measuring eyebrow hairs/cm2 and calculating mean diameter of hair. Safety evaluation- Subjects were assessed for any symptom or sign of dermatitis or allergic event, rated as mild, moderate, or severe.
Statistical Analysis
Comparison of the change in global photographic score (Subject’s satisfaction) before and after Minoxidil treatment was done. Also the diameter and number of eyebrow hairs/cm2 areas between pre and post-treatment determined. SPSS version 17.0 was used for statistical analysis. Determination of p-value less than 0.05 indicated significant differences.
Results
The demographic data is shown in [Table/Fig-1]. Before treatment with Minoxidil the average hair shaft diameter was 0.034±0.0057 mm and density was 17±5.03 hairs/cm2. After treatment this improved to 0.07±0.0045 mm and 30±7.03 hairs/cm2 respectively.
Subject characteristics of patients at baseline and post treatment.
| Subject characteristics | Number |
|---|
| Gender |
| Female | 17 |
| Male | 5 |
| Mean eyebrow hair diameter in mm |
| Before | 0.034 |
| After | 0.070 |
| Mean number (hair) per cm square |
| Before | 17 |
| After | 30 |
| The Global photographic score before treatment | -2.1 |
| The Global photographic score after treatment | 2.3 |
| GEBA scale before treatment | 1.45 |
| GEBA scale after treatment | 3.18 |
| Side effects | 22.78% (5/22) |
The global photographic score was -2.1±0.76 before treatment, increased to -1.3±0.89 at 4 weeks and reached a peak of 2.3±0.55 at 16 weeks [Table/Fig-2,3].
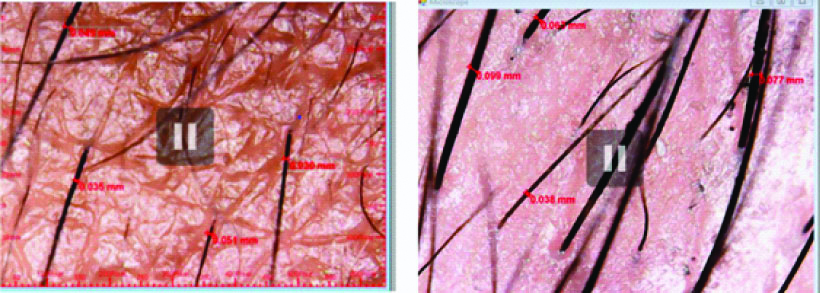
Folliscope image of same patient and of same region showing eyebrow density and mean hair shaft diameter before and after treatment.
Results of paired t-test.
| Parameter | Pre treatment | Post treatment | Average of differences | Sd | Normality p-value | t-test |
|---|
| Hair diameter | 0.034 | 0.07 | 0.0360000 | 0.00426503 | 0.002166 | 39.590570 |
| Hair density | 17 | 30 | 13.00000 | 7.050836 | 0.02055 | 8.647968 |
| Global photographic score | -2.1 | 2.3 | 4.331818 | 0.925340 | 0.06205 | 21.957364 |
| GEBA scale | 1.45 | 3.18 | 1.727273 | 0.767297 | 0.0002759 | 10.558660 |
Five patients were found to be suffering from minor adverse effect [Table/Fig-4].
Side effects encountered.
| Side effects | Number of patient affected | Steps taken |
|---|
| Local redness with burning | 2 | Local application of cold pack |
| Itching | 1 | Local application of antihistaminics (Caladryl Lotion) |
| Dryness | 2 | Moisturising cream |
Discussion
The present study suggests minoxidil as a good therapeutic agent for the treatment of eyebrow hair loss.
A similar 16-weeks study conducted by Worapunpong N and Tanglertsampan C, on 40 subjects depicted that 1% minoxidil showed significant outcome for eyebrow diameter, global photographic assessment, eyebrow hair count, and patient’s satisfaction in placebo. Both grouped showed similar side effects [7]. Riahi RR, Cohen PR, describes promising result with Minoxidil in a 60-year-old woman [8]. Results were same as of present study.
In a study conducted by Hasanzadeh H et al., no participants suffered any skin burning, itching, erythema, swelling, or scaling with minoxidil 5% topical foam [9]. Adverse effects after minoxidil 2% topical solution on the scalp (such as itching, dryness, and redness) were observed in 7% of patients. Here propylene glycol is an important ingredient in the sensitivity of irritated skin. As the foam is free of propylene glycol, so side effects are less. In present study 5 adverse events were observed.
Lee S et al., conducted a randomised, double blind, placebo controlled, spilt-face comparative study involving 40 patients with eyebrow hypotrichosis to evaluate the efficacy of topical Minoxidil 2% lotion [10]. The Minoxidil-treated group was shown to be superior to the placebo group. Results are similar to present study.
Limitation(s)
It was a single centre study and was not a blinded study. No placebo- control groups were available for comparison.
Conclusion(s)
Minoxidil (2%) is a good treatment modality for hypotrichosis of eyebrows.
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Sep 10, 2019
Manual Googling: Dec 05, 2019
iThenticate Software: Jan 11, 2020 (13%)
[1]. Velez N, Khera P, Eyebrow loss: Clinical review. English JC 3rdAm J Clin Dermatol 2007 8:337-46.10.2165/00128071-200708060-0000318039016 [Google Scholar] [CrossRef] [PubMed]
[2]. Chanasumon N, Sriphojanart T, Suchonwanit P, Therapeutic potential of bimatoprost for the treatment of eyebrow hypotrichosisDrug Des Devel Ther 2018 12:365-72.10.2147/DDDT.S15646729503529 [Google Scholar] [CrossRef] [PubMed]
[3]. van der Velden EM, Drost BH, Ijsselmuiden OE, Baruchin AM, Hulsebosch HJ, Dermatography as a new treatment for alopecia areata of the eyebrowsInt J Dermatol 1998 37(8):617-21.10.1046/j.1365-4362.1998.00540.x9732013 [Google Scholar] [CrossRef] [PubMed]
[4]. Orasan MS, Roman II, Coneac A, Muresan A, Orasan RI, Hair loss and regeneration performed on animal modelsClujul Med 2016 89(3):327-34.10.15386/cjmed-58327547051 [Google Scholar] [CrossRef] [PubMed]
[5]. Ortiz AE, Alster TS, Rising concern over cosmetic tattoosDermatol Surg 2012 38:424-29.10.1111/j.1524-4725.2011.02202.x22093105 [Google Scholar] [CrossRef] [PubMed]
[6]. Greenland S, Robins JM, Estimation of a common effect parameter from sparse follow-up dataBiometrics 1985 41:55-68.10.2307/25306434005387 [Google Scholar] [CrossRef] [PubMed]
[7]. Worapunpong N, Tanglertsampan C, Treatment of eyebrow hypotrichosis with 1% minoxidil lotion: A prospective, randomized, double-blind, placebo-controlled trialJ Med Assoc Thai 2017 100(5):573 [Google Scholar]
[8]. Riahi RR, Cohen PR, Topical treatment of eyebrow hypotrichosis with bimatoprost 0.03% solution: Case report and literature reviewCureus 2018 10(5):e2666(May 21, 2018)10.7759/cureus.2666 [Google Scholar] [CrossRef]
[9]. Hasanzadeh H, Nasrollahi SA, Halavati N, Saberi M, Firooz A, Efficacy and safety of 5% minoxidil topical foam in male pattern hair loss treatment and patient satisfactionActa Dermatovenerol APA 2016 25:41-44H.(scale)10.15570/actaapa.2016.1227695865 [Google Scholar] [CrossRef] [PubMed]
[10]. Lee S, Tanglertsampan C, Tanchotikul M, Worapunpong N, Minoxidil 2% lotion for eyebrow enhancement: A randomized, double-blind, placebo-controlled, spilt-face comparative studyJ Dermatol 2014 41:149-52.10.1111/1346-8138.1227524471459 [Google Scholar] [CrossRef] [PubMed]