Normal ovarian function is integral to reproduction in females. Reproductive health affects women from conception to birth, through adolescence to old age. The process of oocyte atresia begins before birth and it continues throughout reproductive years until menopause. But if it reaches before the age of 40 years the condition is known as POF [1].
The disease is characterised by hypergondotropic (FSH>40IU/L) hypoestrogenic (E2<10 pg/mL) and amenorrhea in women under the age of 40 years [1]. Despite various aetiologies described most cases remain idiopathic. The biomarkers that have been used to confirm diagnosis of POF are the hormonal levels of FSH, LH and E2 but they fail to detect the disease at an early stage. The most common cause underlying the idiopathic POF is genetic [2].
POF is proposed to be genetically heterogenous and genetic aetiology accounts for 20-25% cases of POF, X chromosome aberrations being the most common [3]. Several new genes have been identified in distal portion of Xq26 which are proposed to be associated with POF, these genes are HS6ST2, GPC3 and E2F. Interaction of various genes affects the phenotype.
HS6ST2 is one of the candidate genes of POF present in critical region at Xq26.2. This gene encodes an enzyme HS6ST which catalyses the transfer of sulphate from 3′-phosphoadenosine 5′-phosphosulphate to position 6 of the N-sulphoglucosamine residue of Heparan Sulphate (HS). HS-Proteoglycans (HSPG) are present on the cell surface, extracellular matrix and basement membranes and are known to regulate cell proliferation, differentiation, adhesion, migration, and ovarian folliculogenesis [4]. HS6ST2 mutations in POF patients have not been studied so far. HS6ST2 mutations may disturb HS-GF-protein complex mediated signaling and folliculogenesis. This may lead to premature failure of ovarian function.
As POF has genetic heterogeneity, it is expected to be caused by various unidentified novel gene variations. Finding novel gene alterations will enhance the understanding of molecular pathways involved in ovarian physiology. Early identification of genetic alterations helps in early diagnosis of POF. This will help in predicting the likelihood of early menopause and allow these women to opt for embryo preservation techniques or planning of early pregnancy.
Genetic alterations or mutations are called as the permanent changes in the DNA either in germ cells or somatic cells. The change can be an alteration in the nucleotide sequence in coding portions of the DNA which may alter the amino acid sequences of proteins, or a change in non-coding regions of DNA which has the potential for changing expression of the gene. If present in the germ cells, mutations can cause conception of a foetus suffering from defects since birth. Mutations can either be categorised on the basis of gain or loss of function or as chromosomal and DNA-based mutations. Insertion, deletion, translocation are the various types of mutations [5].
The development of new laboratory technologies is needed for accurate understanding of the genome and genetic pathologies. For identification of a disease, molecular analysis can be a great tool. The detection may involve analysis of known mutation or identification of unknown mutations by primary screening which depends upon the size of the gene of interest and the availability of resources.
The SSCP has been used to study gene mutations in ssDNA. It can identify deletion, insertion or rearrangements in amplified DNA. SSCP can detect a single nucleotide change in PCR-amplified product. It is based on the principle that subtle nucleic acid change affects the migration of ssDNA fragment and, therefore, results in visible mobility shifts across a non-denaturing polyacrylamide gel [6]. The differential mobility of test and control sample is suggestive of mutations that can further be sequenced to determine the nucleotide change.
Sanger sequencing involves DNA polymerase for selectively incorporating dideoxynucleotides during in vitro DNA replication [7]. DNA strands can be sequenced directly using sequencing machines and are analysed using sequence analyser software. DNA is first amplified by fluorescent methods and subjected to electrophoresis. The fluorescence signals produced are converted into electronic signals and are analysed through software to produce a coloured graph (chromatogram/electropherogram) that has peaks of different colours specific for a particular nucleotide. The sequence produced is aligned with the reference gene sequence and compared for any base alterations. Even single nucleotide changes may be associated with genetic disorders [8]. Therefore, aim of the study was to evaluate the parameters for detecting HS6ST2 gene mutations in patients of idiopathic POF by SSCP and to confirm the results by Sanger sequencing.
Materials and Methods
A cross-sectional study was planned by including 25 cases of POF which were further screened for evaluation of idiopathic cases. Corresponding to the number of 10 idiopathic cases identified, 10 controls were included. Remaining 15 cases were excluded from the study based on abnormal findings during evaluation. The cases and controls (age<40 yrs) were selected from Outpatient Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, New Delhi, India over 12 months period between February 2017- February 2018. Protocol of the study was approved by the Institute Ethics Committee (IECPG-40/16-2-2017) and written informed consent was obtained from all enrolled cases and controls.
Selection Criteria for Cases
Inclusion criteria: Women below the age of 40 years with secondary amenorrhea, high Follicle Stimulating Hormone (FSH) level (>40 IU/L), low Estradiol (E2) level (<10 pg/mL) measured twice at an interval of 4-6 months.
Exclusion criteria: Cases with known aetiological factors of POF such as genetic diseases, autoimmune disorders, iatrogenic causes, hypothalamic or pituitary disorders were excluded from the study.
Selection Criteria for Controls
Women less than 40 years of age with normal menstrual cycle and reproductive history with at least one live birth; FSH levels upto 10 IU/L and E2 >10 pg/mL.
Out of the total 25 patients of POF recruited for the study, only 10 were idiopathic. Corresponding to 10 cases, 10 normal controls were included. Remaining 15 patients were having some systemic problems therefore were not taken in the study. So, there were total 10 cases and 10 controls included in the study.
Initial evaluation of POF patients: A pre-tested questionnaire was prepared to collect relevant clinical information [Table/Fig-1] and consent of each subject enrolled for the study. [Table/Fig-1] highlights the minimum initial evaluation done in a POF patient.
Initial evaluation of POF patient.
| Initial evaluation | Relevance |
|---|
| Relevant history |
| Menstrual history: Last menstrual period, frequency, duration | Primary amenorrhea /secondary amenorrhea/oligomenorrhea |
| Obstetric history: Live births/ abortions | Primary/secondary infertility |
| Menopausal symptoms: Hot flushes, night sweats, sleep disturbances, mood fluctuations, decreased libido, vaginal dryness, dyspareunia | Estrogen deficiency |
| Family history: Early menopause Autoimmune disordersMale mental retardation | Familial POFAutoimmune POF Fragile X syndrome |
| Medical history: History of fracturesPrior pelvic surgery, irradiation, chemotherapy Infections (mumps, tuberculosis, varicella) Liver disorders, diabetes, bone pain, anaemia | Osteoporosis (estrogen deficiency)Iatrogenic cause of POF, Infectious cause of POFNon-endocrine causes of POF |
| General well-being: Fatigue, cold intolerance, constipation, dry skin, weight gain, hoarseness, swelling in neck, premature graying of hair, slow heart rate | Autoimmune hypothyroidism (Hashimoto’s thyroiditis) |
| General well-being: Anxiety, irritability, tremors, heat intolerance, moist skin, bulging of eyes, palpitations, swelling in neck | Autoimmune hyperthyroidism (Grave’s disease) |
| General well-being: Dizziness/blurred vision/syncope on standing up (postural hypotension), skin hyperpigmentation, unexplained weakness, salt craving, hypoglycemia | Autoimmune adrenalinsufficiency (Addison’s disease) |
| Physical examination general |
| Short stature, webbed neck, short metacarpals, wide carrying angles, low set ears, low posterior hair line | Turner syndrome |
| Narrowing of eye opening, drooping of eyelids | Blepharophimosis |
| Goitre, exophthalmos, bradycardia/tachycardia, premature graying of hair, vitiligo | Autoimmune thyroid dysfunction |
| Skin hyperpigmentation, decreased axillary and pubic hair, postural hypotension, vitiligo | Autoimmune adrenal insufficiency |
| Nail dystrophy, fungal infections of mouth, scalp, skin (mucocutaneous candidiasis) | Autoimmune polyglandular syndrome |
| Butterfly pattern facial rash (malar rash), patchy hair loss (alopecia areata) | Lupus |
| Pelvic examination |
| Atrophic vaginitisSmall or barely palpable ovaries Large ovaries | Estrogen deficiency Ovarian failure Autoimmune oophoritis |
Sample collection: Total 10 mL of subcutaneous venous blood was collected under aseptic conditions and was allowed to coagulate at room temperature (3 mL of blood in heparin vial for interphase and metaphase culture, 5 mL of blood in EDTA vial for DNA extraction, 2 mL of blood in EDTA vial for biochemical analysis). After 30 minutes, samples were centrifuged at 3750 rpm for 10 minutes. Serum was then collected in serum vials using 1 mL micropipette. This was stored at -80°C till further analyses.
Plan of the Study
The methodology was planned in three parts:
In the first part, cases enrolled in the study based on inclusion criteria were further evaluated for excluding possible underlying aetiological factors. Initial laboratory analysis included: FSH, Luteinizing Hormone (LH), E2, Antimullerian Hormone (AMH), inhibin B, triiodothyronine (T3), tetraiodothyronine (T4), Thyroid Stimulating Hormone (TSH), cortisol and prolactin. Antithyroglobulin (Tg), antithyroperoxidase (TPO) and anti-Cyclic Citrullinated Peptide Antibody (CCP) profiling was done to rule out autoimmune aetiology.
In the second part, mutation analysis of HS6ST2 gene was carried out by using SSCP in the selected cases that were found apparently idiopathic.
In the third part, case samples were processed for direct sequencing (Sanger) for confirmation of the results of SSCP.
Part I
A: Clinical and Laboratory Parameters
Estimation of serum levels of FSH, LH, E2, T3, T4, TSH, AMH, inhibin, prolactin, cortisol, antiTPO, antiTg, antiCCP. The hormonal analysis was done in CRIA facility at AIIMS, New Delhi using Architect assay kit.
B: Cytogenetics (FISH and Karyotyping)
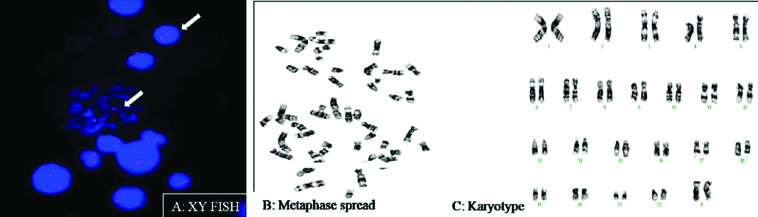
XY-Flourescent In-Situ Hybridization (FISH) and karyotyping was done to study chromosomal alterations if present [Table/Fig-2]. Standard protocols were followed.
a) Interphase and Metaphase cells showing two (red) signals for X-chromosome (FISH); b) Metaphase spread; c): Normal Karyotype
Part II
DNA isolation was done from 5 mL blood samples of cases and controls by manual method (Miller’s method). For 6 exons of HS6ST2 gene, 10 primers were designed by NCBI Primer-Blast tool. Primers were synthesised by IDT (Integrated DNA Technologies).
The DNA samples were then amplified by PCR and processed further for SSCP and sequencing. The details of 10 primers synthesised are given in [Table/Fig-3].
Details of primers designed.
| Sl. No. | Primer sequence (5′---3′) | Exon | Primer length | Product length (bp) |
|---|
| 1 | Forward primers5′-ACCAAATGCATGTCTGGCCT-3′Reverse primers5′-AACTGGCCGAACTGTCAAGT-3′ | 1 | 2020 | 280 |
| 2 | Forward primers5′-TTCAAGCTCGCTCGTGATCC-3′Reverse primers5′-TGGAATCCGTGAGACACACC-3′ | 2 | 2020 | 237 |
| 3 | Forward primers5′-AGCTGCGGGATTGACGTAAC-3′Reverse primers5′-AGGTCATCGCCCTTGATGTC-3′ | 3 | 2020 | 294 |
| 4 | Forward primers5′-GCTCTTAAAGCACAATGGAAAACT-3′Reverse primers5′-CTGAAATTAAACGGACAGGCAACT-3′ | 4 | 2424 | 209 |
| 5 | Forward primers5′-ATGCAGGCTGGCCTTGAG-3′Reverse primers5′-ACAAAAGGAGGACAGCACTTGA-3′ | 5 | 1822 | 250 |
| 6 | Forward primers5′-GTGTCCCGGTACTTGAGTGAG-3′Reverse primers5′-TACCAGGGTCAGGTCGGAGA-3′ | 6 | 2120 | 219 |
| 7 | Forward primers5′-GAAGCACATGGCGTTCTTCG-3′Reverse primers5′-CGTTGTTCCTGACGCTTTCG-3′ | 6 | 2020 | 279 |
| 8 | Forward primers5′-CAGCAATGGCACCAACGACT-3′Reverse primers5′-CTGGTTTGGCTTTCGGATTTCA-3′ | 6 | 2022 | 299 |
| 9 | Forward primers5′-TCAAGCATCATGATTCCGGGC-3′Reverse primers5′-CCCAAATACCCAGCTTCTTGTC-3′ | 6 | 2122 | 236 |
| 10 | Forward primers5′-AAGCATCATGATTCCGGGC-3′Reverse primers5′-AAATACCCAGCTTCTTGTCCAG-3′ | 6 | 1922 | 231 |
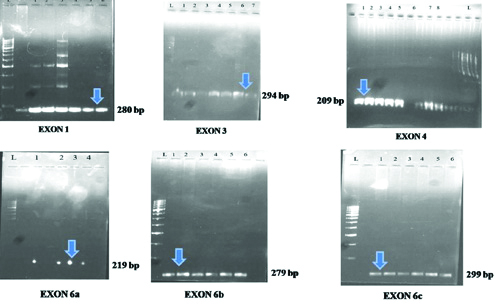
After DNA isolation, primer designing and synthesis, PCR amplification of 10 cases and control DNA samples were done as per the standard protocols. [Table/Fig-4] shows the master mix details. This was followed by agarose gel electrophoresis to confirm the formation of primer bands. Electrophoresis bands were well-formed in primers of exon 1,3, 4 and 6 [Table/Fig-5]. These primers were further processed for SSCP.
| Components | Volume |
|---|
| Forward primer | 0.5 μL |
| Reverse primer | 0.5 μL |
| Deoxyribonucleotide triphosphate | 0.5 μL |
| Taq buffer | 2.5 μL |
| Taq Polymerase | 5 μL |
| DNA | Volume adjusted for concentration 100 ng/μL |
| Tris EDTA | Volume made upto 25 μL |
Electrophoresis results on agarose gel for exon 1, 3, 4, 6a, 6b and 6c. (“L” refers to the DNA ladder, PCR samples were loaded in the wells labelled numerically; arrow shows the location of bands of the corresponding exon).
SSCP: After the primer band confirmation, the PCR amplified products were processed further for SSCP.
Preparation of SSCP Gel
The [Table/Fig-6] shows the composition of 10% polyacrylamide gel (10 mL). The gel was dissolved completely and was kept at -200°C till further used.
Components of polyacrylamide gel.
| Components | Volume |
|---|
| Acrylamide solution | 9.9 mL |
| Distilled water | 12.3 mL |
| 1 X Tris borate EDTA buffer | 7.5 mL |
| Ammonium persulphate | 300 μL |
| Tetramethylethylenediamin | 12 μL |
Steps of SSCP
The SSCP analysis was carried out using Bio-Rad Protein II xi Cell vertical gel electrophoresis unit (Bio-Rad laboratories).
The two glass plates were washed thoroughly using tap water and detergent.
The gel sandwich was sealed at bottom using 10 mL of 12% gel mix. The solution mixed with 100 μL APS and 10 μL TEMED was injected between the two glass plates and allowed to polymerize.
Once polymerized, the assembled gel sandwich was placed in casting stand. The 10% native PAGE gel mix (30 mL) was filled from upper side of gel sandwich and was kept undisturbed at least for 45 minutes for polymerisation.
After polymerisation, the wells were flushed with 1X TBE buffer. The gel sandwich was placed in electrophoresis tank. The electrophoresis tank was filled with 1X TBE. Ice cooled water circulation with electric pump was applied to central cooling core of assembly.
About 5 μL PCR product and 7 μL of a formamide mix was prepared in PCR tube and denatured at 95°C for 10 minutes in the PCR machine. After denaturation, the samples were immediately kept in ice-chilled box.
The samples were loaded on the 10% acrylamide: bis-acrylamide (29:l) gel with gel loading tip and immediately electrophoresis was performed in 1 X Tris borate (pH 8.3)-EDTA buffer at 200 volts for 6 hours.
After completion of the electrophoresis the plates were removed and the gel was subjected to silver staining to visualise the SSCP band patterns.
Silver Staining
9. The gel was immersed in a tray filled with fixative (100% ethanol+0.5% acetic acid+ mili Q;500 mL) for at least 10 minutes for fixing DNA bands in gel so as to prevent diffusion of the DNA bands.
10. The fixative was decanted and then freshly prepared 0.1% silver nitrate was added.
11. The gel was stained for 15 minutes in silver nitrate solution with constant shaking in dark.
12. Then the gel was rinsed with mili Q for 5 minutes to wash off the extra silver nitrate.
13. Mili Q was decanted quickly from the tray and 500 mL of 1.5% NaOH and formaldehyde were added and left for the appearance of bands.
Once the bands were visible, the solution was removed and 0.75% of sodium carbonate solution was added.
Part III
Sanger sequencing: A 15 μL of amplified PCR products of 10 patients were outsourced for sequencing with concentration >80 ng/μL each. Also, 30 μL each (for 10 reactions) of forward and reverse primers of all the 6 exons were sent to “Agrigenome Lab” for sequencing. The details of steps of sequencing have been simplified according to the protocol (BigDye® Terminator v3.1 Cycle Sequencing Kit, Applied Biosystems) from the lab. Further analysis of results was done by using “Chromas Pro” for alignment. ClustalW2 was the other program used for multiple sequence alignment. The analysis was confirmed by NCBI Blast tool.
Statistical Analysis
Statistical analysis was carried out using Stata 9.0 data analysis software. Clinical and laboratory parameters of cases and control group were compared statistically by using two sample t-test and two sample Wilcoxon rank sum (Mann-Whitney) test. The values obtained were expressed as mean±SD along with the p-value.
Results
Part I
A: Clinical and Laboratory Parameters
A total of 25 women with secondary amenorrhea and age less than 40 years were recruited as cases. Ten cases were selected based upon exclusion by clinical and laboratory parameters. Cases with normal medical and surgical history, secondary amenorrhea, elevated levels of FSH, LH, decreased levels of E2, normal levels of T3, T4, TSH, prolactin, anti Tg, anti TPO, anti CCP and USG findings suggestive of POF were selected for the study. The study also included 10 healthy fertile women with normal hormonal profile as the control group [Table/Fig-7].
Laboratory parameters of controls recruited in the study.
| Sl. No. | Age (yrs) | FSH (3.03-8.08 mIU/mL) | LH (1.80-11.78 mIU/mL) | E2 (21-251 pg/mL) | AMH (<0.15 ng/mL) | Inhibin B (<10 pg/mL) | TSH (0.35-4.94 uIU/mL) | T3 (0.58-1.59 ng/mL) | T4 (4.87-11.72 μg/dL) | Anti Tg (<4.11 IU/mL) | Anti CCP (<5.0 U/mL) | Anti TPO (<5.61 IU/mL) | Cortisol (3.7-19.4 μg/dL) | Prolactin (5.18-26.53 ng/mL) |
|---|
| 1 | 26 | 4.18 | 1.85 | 46 | 2.14 | 123.14 | 1.63 | 0.75 | 6.42 | 0.73 | 0.5 | 0 | 6.7 | 16.23 |
| 2 | 24 | 7.03 | 4.77 | 60 | 3 | 131.67 | 3 | 0.87 | 5.76 | 2.06 | 0.5 | 0.82 | 7.4 | 10.34 |
| 3 | 29 | 6.6 | 2.08 | 59 | 3.12 | 34.93 | 2.57 | 0.9 | 7.31 | 2.38 | 0.6 | 0.2 | 3.2 | 11.64 |
| 4 | 26 | 7.44 | 2.91 | 32 | 1.3 | 54.21 | 1.14 | 1.21 | 7.36 | 2 | 0.5 | 0.05 | 8 | 16.52 |
| 5 | 29 | 6.14 | 4.02 | 52 | 2.53 | 122.14 | 2.19 | 0.98 | 6.32 | 2.59 | 0.5 | 0 | 7.3 | 2.90 |
| 6 | 33 | 4.57 | 3.58 | 36 | 2.65 | 91.65 | 1.4 | 1.12 | 7.65 | 1.91 | 0.5 | 0 | 7.3 | 6.42 |
| 7 | 24 | 7.23 | 2.43 | 30 | 2.78 | 133.67 | 4.2 | 1 | 7.33 | 1.81 | 0.5 | 0.06 | 6.7 | 5.86 |
| 8 | 26 | 6.02 | 2.39 | 35 | 3.31 | 115.3 | 2.6 | 1.23 | 7.84 | 2.23 | 2.2 | 0.76 | 4.7 | 14.52 |
| 9 | 26 | 5.16 | 2.03 | 44 | 2.34 | 130.5 | 0.9 | 1.09 | 7.75 | 1.28 | 0.5 | 8.86 | 8.4 | 13.82 |
| 10 | 24 | 5.15 | 4.21 | 63 | 2.56 | 89 | 1.54 | 1.1 | 6.17 | 1.24 | 0.5 | 0.54 | 9.1 | 6.85 |
[Table/Fig-8,9] show the comparison of mean age and hormone levels between cases and controls. There was no statistical difference between age of the controls and cases. The levels of FSH, LH were higher and levels of E2, AMH, inhibin were lower in cases as compared to controls and the difference was statistically significant. The difference between the levels of T3, T4, TSH, cortisol, prolactin, anti Tg, anti TPO, anti CCP between case and controls was found to be statistically insignificant.
Comparison of age between controls and cases.
| Group I (Control) | Group II (Cases) | p-value |
|---|
| Age | 26.7±2.8 | 29.8±6.8 | 0.2013 |
(Cases and control group were compared statistically by using two sample t-test, p-value<0.05 was significant)
Comparison of hormonal levels between controls and cases.
| Variable | Group I (Control) | Group II (Cases) | p-value |
|---|
| p50 (Min-Max) | Mean±S.D | p50 (Min-Max) | Mean±S.D | |
| FSH | 2.85 | 5.95±1.14 | 60.12 | 58.5±14.8 | 0.0015 |
| LH | 2.67 | 3.02±1.04 | 35.19 | 30.79±11.8 | 0.0002 |
| E2 | 45 | 45.7±12.32 | 10 | 13.54±6.5 | 0.0001 |
| AMH | 2.6 | 2.57±0.57 | 0.15 | 0.10±0.06 | 0.0001 |
| Inhibin B | 118.7 | 102.6±34.6 | 10 | 9.93±0.21 | 0.0001 |
| TSH | 1.91 | 2.11±1.00 | 1.74 | 2.44±1.37 | 0.5452 |
| T3 | 1.04 | 1.02±0.15 | 1.13 | 1.12±0.12 | 0.1207 |
| T4 | 7.32 | 6.99±0.74 | 7.61 | 7.72±1.26 | 0.2263 |
| Anti Tg | 1.95 | 1.82±0.57 | 2.63 | 4.60±7.24 | 0.1506 |
| Anti CCP | 0.5 | 0.68±0.53 | 0.5 | 0.59±0.48 | 0.3305 |
| Anti TPO | 0.13 | 1.12±2.73 | 0.48 | 0.42±0.30 | 0.2886 |
| Prolactin | 10.99 | 10.5±4.79 | 8.4 | 10.85±7.74 | 0.9035 |
| Cortisol | 7.3 | 6.88±1.74 | 6.7 | 7.64±2.96 | 0.8499 |
(Cases and control group were compared statistically by using two sample Wilcoxon rank sum (Mann-Whitney) test. p-value <0.05 was significant)
B: Cytogenetics (FISH and Karyotyping)
XY- FISH was carried out to check for any aneuploidy in the recruited patients. It was carried out using X chromosome specific centromeric probes. The genetic makeup of all the cases was normal i.e., XX. Conventional cytogenetics (karyotyping) was also performed for all the patients. Karyotyping results in all the patients were normal.
The present study could exclude 15 patients based on the abnormal values of hormones and the antibodies (anti Tg, anti TPO, anti CCP), normal/unavailable USG reports.
Part II
A: PCR and SSCP
Ten primer sets (forward, reverse) were designed for 6 exons of gene HS6ST2 and PCRs were carried out with DNA sample of 10 cases and controls. The experiments for PCR standardisation were successful for 6 primers. [Table/Fig-10] shows steps taken for standardising PCR cycle.
PCR cycle standardisation.
| Denaturation temperature | Annealing temperature with no. of cycles | Extension temperature | PCR results |
|---|
| 95°C for 10 minutes | 60°C for 1 minute- 40 cycles | 72°C- 7 minutes | No bands on agarose gel |
| 94°C for 5 minutes | 60°C for 48 seconds- 25 cycles | 72°C- 5 minutes | No bands on agarose gel |
| 94°C for 5 minutes | 54°C for 48 seconds- 35 cycles | 72°C for 5 minutes | No bands on agarose gel |
| 94°C for 5 minutes | 54°C for 48 seconds- 35 cycles | 72°C for 5 minutes | Bands were seen on agarose gel |
To check for the PCR results, electrophoresis was carried out on 1% agarose gel and primers for exons 1, 3, 4 and 6 of the HS6ST2 gene showed bands for the designed PCR cycle [Table/Fig-5]. The cycle was set for 6 primers of exon no. 1, 3, 4 and 6 (primer a, b and c). [Table/Fig-11] shows the details of the primers standardised after agarose gel electrophoresis.
Details of 6 primers standardised for SSCP.
| Sl. No. | Primer sequence (5′---3′) | Exon | Primer length | Product length (bp) |
|---|
| 1 | Forward primers5′-ACCAAATGCATGTCTGGCCT-3′Reverse primers5′-AACTGGCCGAACTGTCAAGT-3′ | 1 | 2020 | 280 |
| 2 | Forward primers5′-AGCTGCGGGATTGACGTAAC-3′Reverse primers5′-AGGTCATCGCCCTTGATGTC-3′ | 3 | 2020 | 294 |
| 3 | Forward primers5′-GCTCTTAAAGCACAATGGAAAACT-3′Reverse primers5′-CTGAAATTAAACGGACAGGCAACT-3′ | 4 | 2424 | 209 |
| 4 | Forward primers5′-GTGTCCCGGTACTTGAGTGAG-3′Reverse primers5′-TACCAGGGTCAGGTCGGAGA-3′ | 6 (a) | 2120 | 219 |
| 5 | Forward primers5′-GAAGCACATGGCGTTCTTCG-3′Reverse primers5′-CGTTGTTCCTGACGCTTTCG-3′ | 6 (b) | 2020 | 279 |
| 6 | Forward primers5′-CAGCAATGGCACCAACGACT-3′Reverse primers5′-CTGGTTTGGCTTTCGGATTTCA-3′ | 6 (c) | 2022 | 299 |
Further with PCR amplified products of 4 exons using 6 primer pairs, SSCP was performed to study the mobility pattern of the DNA during vertical gel electrophoresis.
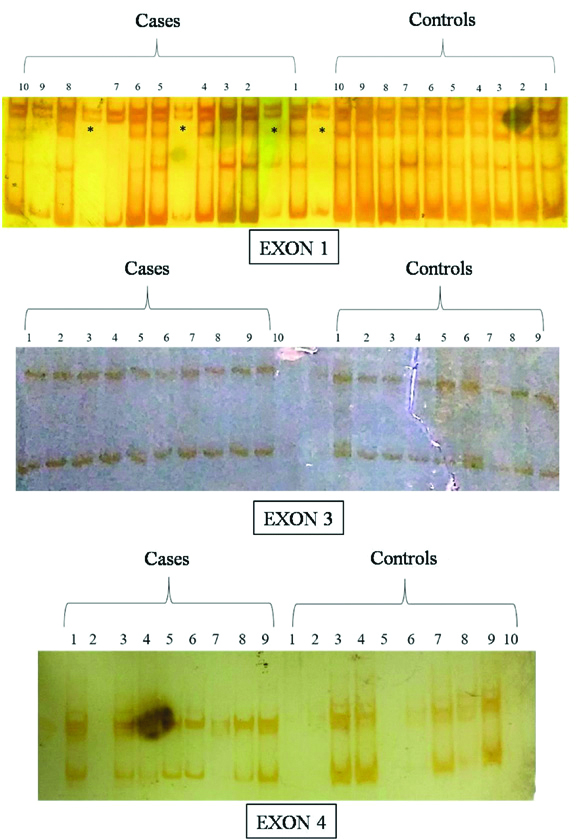
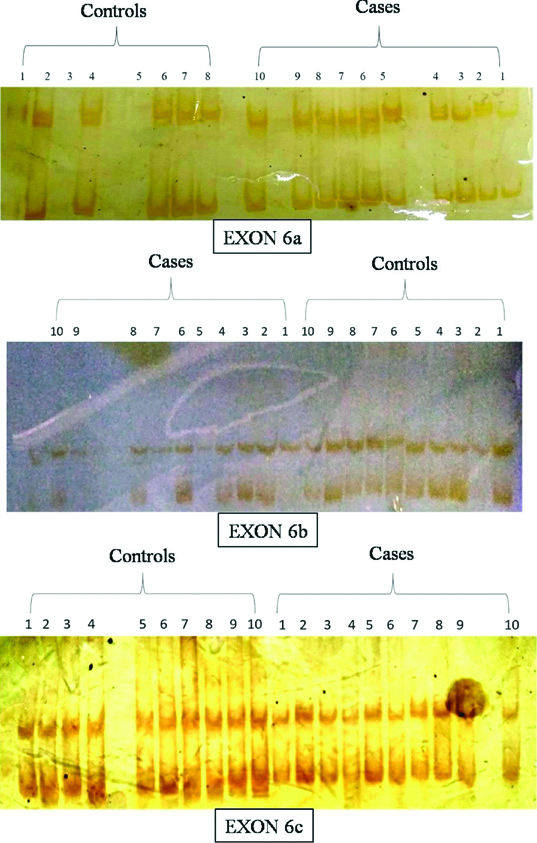
SSCP results showed no difference in the pattern of bands formed after silver staining between cases and controls, indicating absence of any mutation in the gene for these 4 set of exons [Table/Fig-12,13].
SSCP bands in the vertical non-denaturing gel electrophoresis of exon 1, 3 and 4 PCR products (Exon 1 result of SSCP show extra bands represented by (*) due to overspilling of samples).
SSCP bands in the vertical non-denaturing gel electrophoresis of exon 6a, 6b and 6c PCR products.
Part III
A: Sequencing
To confirm the results obtained from SSCP, the amplified PCR products were outsourced for sequencing. Sequence alignment was done using “Cromas Pro” software and confirmed by NCBI Blast tool. There were evenly spaced well resolved peaks with no or minimal baseline noise. Spacing between the base call letters at top was regular that indicated reliability of data. The case/query sequence was compared base to base with the reference sequence. After analysing the sequence alignment, no mutation was found in all the cases [Table/Fig-14]. The sequencing results confirmed the results obtained from SSCP.
Details of an aligned DNA sequence.
(The sequence in black at the top is the ‘reference sequence’ that the software picks from “NCBI reference sequence of HS6ST2 gene”. Below is the “query sequence” of the case sample corresponding to the primer used. Lowermost is the four coloured chromatogram that represents sequence of case sample)

Discussion
Clinical and Laboratory Parameters
Most common clinical presentation of patients with POF is secondary amenorrhea apart from infertility and menopausal symptoms [9]. In the present study, patients presented with chief complaints of secondary amenorrhea, infertility, hot flushes, sleep disturbances, fatigue, depression etc. Loss in reproductive potential in POF is an irreversible consequence due to failure of ovarian function at the time patients present to the clinics. Ovaries fail to function because of premature follicular depletion due to inherently smaller ovarian reserve or accelerated oocyte depletion as a result of wide spectrum of disorders [10,11]. Folliculogenesis is a highly organised and complex process. Various hormones and growth factors are produced by granulosa and theca cells including inhibin, FOXL2, BMP15 and GDF9, which are regulated by the gonadotropins-FSH and LH secretion. Levels of FSH rise as a feedback mechanism to the declining ovarian function and low E2 levels. More the dismissal of ovarian function, higher the levels of FSH and LH in body. The womenincluded as cases in the study were certainly at ovarian failure stage. To compare the study results of cases, controls were also included. The difference in the levels of both hormones was statistically significant thus ensuring that controls were physiologically normal and cases were in ovarian failure stage.
POF is sometimes referred to early menopause due to high gonadotropin levels, menopausal symptoms and decrease in reproductive potential. There has been gradual change in levels of reproductive hormones such as FSH, LH, E2, AMH and inhibin B as the women advances towards menopause and the time period is referred as menopausal transition. These hormones are the biomarkers of reproductive ageing. FSH is secreted from anterior pituitary and is regulated in part by negative feedback of inhibin B and E2 which is secreted by granulosa cells. Due to ovarian ageing and decline in follicular pool, levels of E2 and inhibin B decline. Hence, there is decrease in negative feedback as a result of which level of FSH rises. AMH and inhibin B are direct markers of the ovarian pool and are produced by early ovarian follicles [12]. With its expression primarily restricted to granulosa cells of preantral and small antral follicles, AMH is involved in primordial follicle recruitment, therefore low levels of AMH represents that this process has slowed down [13]. The cases in the present study also had low or undetectable levels of AMH indicating the depletion of follicles. Levels of AMH are independent of the day of the menstrual cycle and also decrease with age as the follicles undergo atresia with progressing age. This makes AMH a very good marker of fertility decline in POF. Inhibin B is secreted by granulosa cells and the levels decrease in women suffering from POF which was confirmed in the present study. Data till now suggest that levels of FSH and LH rises whereas AMH and inhibin B levels decline as the women advances towards menopause. AMH is the first marker to change followed by FSH and inhibin B levels [14]. High prolactin levels lead to menstrual irregularities and infertility [15]. Cases with normal levels of prolactin were selected in view of excluding hyperprolactinemia as the cause of infertility and menstrual problems.
Autoimmune etiology may contribute in up to 30% cases of POF [16]. Common autoimmune conditions associated with POF are Graves’ disease, Addison’s disease and type 1 diabetes mellitus [17,18]. It was reported in one study that 40% of women with POF were positive for at least one autoantibody, anti-thyroid antibodies were the most common amongst them [19]. The presence of anti-TPO antibodies was also studied in POF with 24% showing positive results [17]. Anti-CCP antibody levels rise in rheumatoid arthritis and are a diagnostic and prognostic tool [20]. Menopause at an early age is one of the predictor of rheumatoid arthritis [21]. Rheumatoid arthritis has been associated with POF [22].
In this study, apparently idiopathic patients of POF were selected based on normal medical history, surgical history, physical examination during initial evaluation and serum levels of T3, T4, TSH, anti-TPO antibody, anti-Tg antibody, anti-CCP, cortisol and prolactin. The difference in the serum levels of these parameters between cases and controls was not statistically significant.
Cytogenetics (FISH and Karyotyping)
POF can also occur as a consequence of genetic abnormality in around 5% of the cases [23]. Genetic abnormalities include insertion, deletion, translocation or can be numerical as in Turner syndrome [24]. Women with Turner syndrome have 45, XO karyotype and initially have normal count of ovarian follicle, which disappear after puberty [10]. Conventional cytogenetic methods are able to identify these genetic abnormalities. Also, in the present study, genetic constitution of patients was identified by FISH and karyotyping and women with normal 46, XX genetic make-up were recruited as cases.
By the initial assessment of clinical and laboratory parameters, probable infectious, iatrogenic, endocrine or autoimmune and aetiologies could be excluded and apparently idiopathic cases of POF were selected.
PCR, SSCP and SANGER SEQUENCING
SSCP is a rapid and reproducible method for detecting mutations (deletions, insertions and rearrangements) in PCR amplified DNA [25]. For better results, fragment size should be between 150-300 bp. Under non-denaturing conditions, ssDNA acquires a unique conformation and even if a single base alteration is present, it can result in a conformational change. This change can be detected by the altered mobility of these ssDNA. The conformation and movement of ssDNA on polyacrylamide gel can be affected by various factors like temperature of the gel, concentration of buffer and presence of denaturing agents in gel [6].
SSCP is an adequate screening technique, recommended as a first approach, useful to identify nucleotide changes in a relatively rapid way [26]. Using SSCP, two novel SNPs exhibiting six genotypes were found [27]. RFLP and SSCP were compared and shown that the latter was more reliable for identifying mutants. Although RFLP identified the mutants but SSCP provided higher resolutions for the identification of different mutations. Although recommended as a reliable and sensitive technique, results of PCR-SCCP are confirmed by sequencing. In the present study also this technique was used as screening method for the detection of mutations if present in the gene of interest. The patient and control amplified DNA was run on non-denaturing PAGE to check for altered pattern of DNA mobility. The absence of difference in the pattern of bands observed after silver staining of the gel indicated probable absence of mutant DNA. SSCP results were further confirmed by sequencing.
Sanger sequencing is used in wide areas of research such as sequencing of variable regions, targeting small genomic regions in a larger number of samples, HLA typing, identification of single disease-causing genomic variants, verification of plasmid sequences. It is also used where NGS fails to yield adequate read coverage/specificity due to high GC content or pseudo genes. The technique is also preferred over NGS in sequencing of single genes and where cost effective sequencing is required for single samples. DNA sequencing produces a chromatogram of 4 differently coloured peaks corresponding to a different DNA base. Good sequence generally starts from base 20-40, little base line noise can be avoided but there should not be double or non-discrete peaks. Once the DNA fragments are sequenced, the sequence is aligned to identify regions of similarity that may indicate functional or structural similarity between two sequences in pairwise sequence alignment or between three or more sequences of similar length in multiple sequence alignment. The difference in the sequence pattern indicates presence of a mutation. Sequence alignment in the present study between reference sequence and case sequence showed no change that indicated absence of HS6ST2 mutations in 10 cases included in the present study.
Limitation(s)
Absence of HS6ST2 mutations in 10 cases certainly does not rule out its contribution in POF as results are to be validated in larger cohorts and with remaining exon sequences.
Conclusion(s)
The present study aimed to evaluate parameters to first identify idiopathic cases of POF and then to standardise SSCP to identify HS6ST2 gene mutations in these patients. HS6ST2 gene that regulates sulfation of HS that is an essential process for regulating HS-protein mediated signaling pathways regulating cell growth and differentiation essential for ovarian folliculogenesis. Moreover, HS6ST2 mutations in POF patients have not been studied so far. As the study was based on candidate gene approach, the results faithfully state absence of mutations in the exons of HS6ST2 gene included in the study. Moreover, this study also confirmed SSCP as a sensitive, rapid and cost effective method to screen unknown mutations in the gene of concern. The study shall be planned in larger cohorts to confirm validity of results. In case if genomic variants are found in more number of studies planned, it will help in developing functional studies in both human cell lines and in vivo mouse models to validate the association of genomic variants in PO.