Granular Cell Tumour Masquerading as Carcinoma Breast
Sadiya Shafi1, Charles Mano Sylus2, Salapathi Shanmugam3, P Darwin4
1 Consultant, Department of Histopathology, Apollo Speciality Hospital, Chennai, Tamil Nadu, India.
2 Senior Registrar, Department of Surgery, Apollo Speciality Hospital, Chennai, Tamil Nadu, India.
3 Junior Consultant, Department of Histopathology, Apollo Speciality Hospital, Chennai, Tamil Nadu, India.
4 Senior Consultant, Department of Surgery, Apollo Speciality Hospital, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sadiya Shafi, Flat No. T1, Door No. 21, Balaji Nagar, 2nd Street, Royapettah, Chennai, Tamil Nadu, India.
E-mail: shajanabasraar@gmail.com
Granular Cell Tumour (GCT) is a rare benign tumour that affects head and neck, the most frequent site being the tongue. GCT with localisation to breast is very rare with an incidence of 4-6%. It is an uncommon cause of breast mass in premenopausal women. The usual clinical presentation is with a painless solitary nodule. But rarely the lesions can be multifocal as a manifestation of a multicentric disease. On clinical and radiological examination, GCT can be confused with infiltrating carcinoma, therefore making its diagnosis challenging for the clinicians, radiologists and pathologists. It is pertinent to differentiate this tumour from other malignant tumours of the breast and avoid misdiagnosing as mammary carcinoma. Definitive preoperative diagnosis helps to prevent unnecessary mastectomy. We report a breast lump in a post-menopausal woman, which was mimicking malignancy clinically and radiologically, but on histopathological examination was diagnosed as a GCT.
Periodic acid schiff diastase, Schwann cell derived neoplasm, S100 protein, Wide local excision
Case Report
A 57-year-old postmenopausal female patient presented with the complaints of lump in the left breast of six months duration with no history of pain or discharge from nipple. There was no family history of carcinoma breast nor was there any significant medical or personal history. On examination, a firm to hard 2 cm mass with restricted mobility, palpable in the left breast at 3’o clock position. No axillary lymphadenopathy noted. A diagnostic sonography done elsewhere revealed a well-defined hyperechoic mass lesion in the left breast with speckled calcification at 3’o clock position, categorised as BIRADS 4B and was suspicious for mammary carcinoma. The other differential considered was fibromatosis.
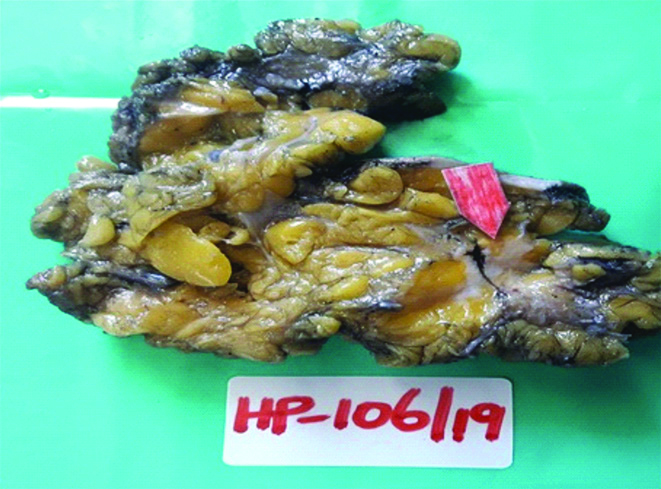
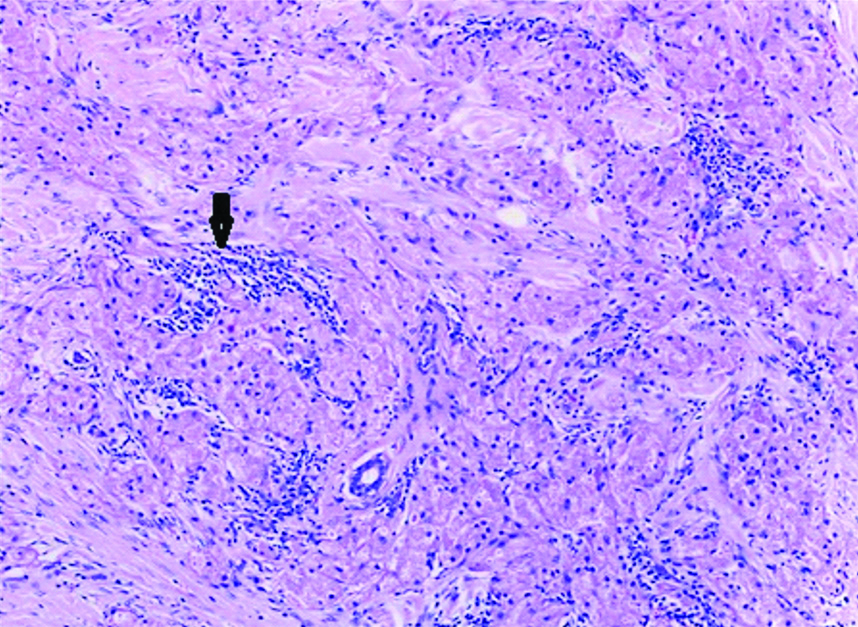
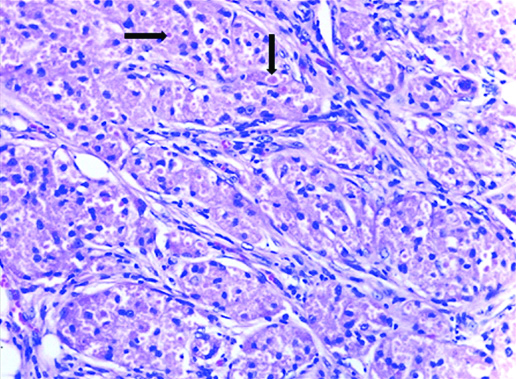
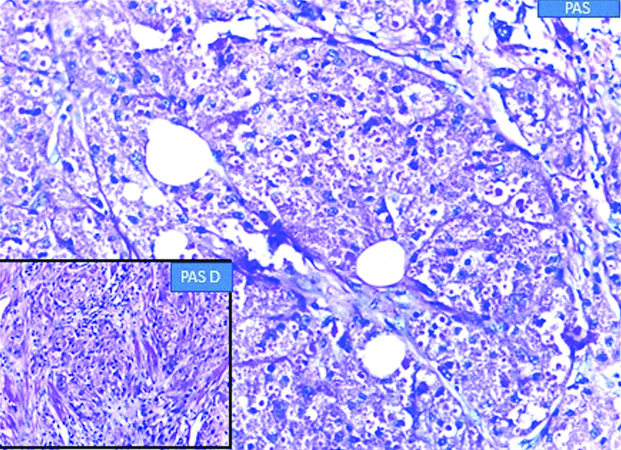
In view of the suspicion of malignancy, a core biopsy was done, which revealed linear cores of breast tissue showing polygonal cells with round nuclei and granular eosinophilic cytoplasm with foci of lymphoid aggregates. Nomitosis, necrosis or spindling were seen. A diagnosis of GCT was rendered. A wide local excision with adequate margins was suggested. Gross examination of the wide local excision specimen showed a greyish white hard lesion measuring 2.0×1.5×1.0 cm situated 1.0 cm below the skin [Table/Fig-1]. The lesion showed a traversing tract representing the previous site of biopsy and the lesion was situated at a distance of 0.3 cm from the closest inked line of resection. Microscopy showed breast parenchyma with a fairly circumscribed benign neoplasm arranged in sheets, nests and ill-defined clusters with stromal lymphoid aggregates [Table/Fig-2]. The tumour showed cells with round to oval darkly stained nuclei showing finely stippled chromatin with conspicuous nucleoli and with moderate to abundant granular eosinophilic cytoplasm with intracytoplasmic inclusion bodies termed Pustolo-ovoid bodies of Milan [Table/Fig-3]. No spindling of cells, mitosis or necrosis seen. Special stain for Periodic Acid Schiff (PAS) and PAS Diastase showed PAS positive intracytoplasmic granules resistant to diastase [Table/Fig-4].
Gross photograph of the wide local excision specimen showing a greyish white hard lesion with a traversing tract representing site of previous biopsy (as depicted by an arrow).
Photomicrograph showing breast parenchyma with a benign neoplasm in sheets, nests, ill-defined clusters and with stromal lymphoid aggregates (as depicted by an arrow) (H&E 10X).
Photomicrograph showing tumour cells with round to oval nuclei showing finely stippled chromatin, inconspicuous nucleoli, moderate granular eosinophilic cytoplasm with Pustulo-ovoid bodies of Milan (as depicted by an arrow) (H&E 40X).
Photomicrograph showing PAS positive intracytoplasmic granules resistant to diastase (as shown in inset).
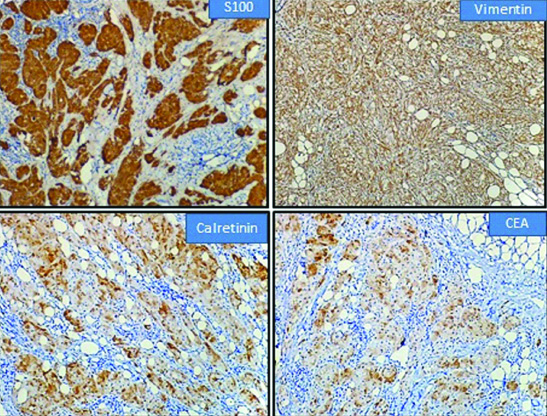
Immunohistochemistry done showed diffuse positivity for S100, Vimentin, CEA and Calretinin [Table/Fig-5] with negativity for ER, PR and CK. Ki-67 proliferation index was 1%. Based on histology, histochemistry and immunohistochemical findings, a diagnosis of GCT was confirmed.
Immunohistochemistry showing positivity for S100, Vimentin, Calretinin and CEA.
Discussion
GCT is an uncommon neoplasm that was first alluded by Weber in 1954. It was termed as Granular cell myoblastoma by Abrikossoff in 1926, since a myogenic origin was suspected. However, in view of the S100 protein positivity and the similarity of the tumour cells to Schwann cells, the researchers proposed that the tumour originated from Schwann cells, though the histogenesis of this tumour is still unknown [1]. In view of the worrisome mammographic and sonographic features [2], these lesions are nearly always biopsied. GCT is benign and the treatment is wide local excision. However, in <1% of cases, a legitimate malignant counterpart exists [3].
GCT is a rare benign neoplasm which occur in viscera, soft tissue, cutaneous sites and is seen predominantly centered over the head and neck and chest wall region. The other anatomic locations include vulva and breast with tongue being commonly involved in middle aged patients. Between 4-6% of GCT are seen in the breast in the middle aged premenopausal women. GCT occurs in male breast with a Male: Female ratio of 1:9 [4]. Among breast cancers, GCT occurs in 1 of every 1000 breast cancers. The youngest reported patient was 14 years [5]. Most of the reported cases are seen in premenopausal women [6]. Present case patient was postmenopausal. A review of literature reveals GCT to be commonly located in upper inner quadrant, maxillary and periareolar region, a possible aetiology for the predelicted quadrant is attributed to the cutaneous sensory distribution of the supraclavicular nerve [6].
About <1% of GCT of the breast are malignant. The most widely used radiological modalities for diagnosis include mammogram and ultrasound scanning. However, on radiology, GCT simulates a growth pattern similar to that of an infiltrating mammary carcinoma and nodular fasciitis. Clinically, it mimics malignancy by exhibiting nipple and/or skin retraction [7].
Wide local excision is sufficient for treatment of GCT. Subtotal and incomplete excision leads to recurrence. A study by Papalas JA et al., with long term median follow-up of 13 patients with GCT of breast focuses on risk of tumour recurrence in patients with excisions having positive and close (<1.0 mm) margins [8]. Our patient had a wide local excision with a 3 mm clearance from the closest inked resected surface.
The characteristic cytomorphological features of GCT are the presence of large granular cells with abundant granular eosinophilic cytoplasm from which the tumour derives its name. The stroma shows lymphoid aggregates and plasma cells with thin walled blood vessels. Nuclei are small and centrally located, hyperchromatic with one or two nucleoli. The cytoplasm shows PAS positive inclusion bodies which are diastase resistant. These intracytoplasmic granules and inclusion bodies which are aggregates of lysosomes are termed pustulo-ovoid bodies of Milian and are rarely seen in cytology smears [9].
The distinction between benign and malignant GCT was proposed by Adeniran A et al., Le BH et al., which included spindling, necrosis, vesicular nuclei with large nucleoli, high nucleo-cytoplasmic ratio, nuclear pleomorphism and increased mitotic activity (more than 2 mitosis/10hpf) [6,10]. The above criteria categorise GCT based on histology into atypical when two out of six criteria are present and malignancy when three or more of the six criteria are seen. A definitive diagnosis of GCT can be made by immunohistochemistry based on diffuse positivity for S100 which supports a Schwann cell origin, CEA and Vimentin, with negativity for cytokeratin and EMA. In present case CEA, S100, Vimentin and Calretinin were positive with negativity for CK, ER and PR.
It is interesting to find positive expression of Calretinin in these tumours which has been recently described. Calretinin, primarily a neuronal protein, when expressed supports the neural derivation of these lesions [11]. In addition to S100 protein, calretenin has been found to be a useful diagnostic marker. Present case was also positive for Calretinin. GCT shows negativity for hormone receptors namely ER and PR. Although oestrogen and progesterone receptors have been thought to play a role in GCT pathogenesis, in most cases, hormone receptors are negative.
The expression of Vimentin distinguishes GCT from mammary carcinoma which shows negativity for Vimentin and from other metastatic tumours of breast such as renal cell carcinoma, melanoma and alveolar soft part sarcoma which show oncocytic or clear cell features [12].
Athough GCT is common in premenopaual women this patient was postmenopausal. Since the radiological findings closely mimicked a mammary carcinoma, a core biopsy proved pertinent in diagnosing the lesion and avoiding an unnecessary mastectomy. The diagnosis was confirmed by immunohistochemistry which also ruled out the other histological differentials as well. The positivity for S100 and Calretinin highlights the neural origin of the tumour.
Conclusion(s)
GCT of the breast is a rare benign neoplasm of the breast in premenopausal women which mimics carcinoma both clinically and radiologically. In BIRADS category 4 or 5 lesions, it has been considered as a part of the differential diagnosis. The treatment option is wide local excision. However, to avoid unnecessary mastectomy and axillary clearance, a preoperative definitive diagnosis with image guided biopsy is mandatory, as done for infiltrating carcinomas. In addition to S100 protein, calretinin expression has been found to be a useful diagnostic marker which supports the neural origin and derivation of these lesions.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: May 20, 2019
Manual Googling: Jun 14 2019
iThenticate Software: Jan 09, 2020 (16%)
[1]. Brown AC, Audisio RA, Regitnig P, Granular cell tumour of the breastSurg Oncol 2011 20(2):97-105.10.1016/j.suronc.2009.12.00120074934 [Google Scholar] [CrossRef] [PubMed]
[2]. Maki DD, Horne D, Damore LJ II, Jones C, Magnetic resonance appearance of granular cell tumour of the breastClin Imaging 2009 33(5):395-97.10.1016/j.clinimag.2008.10.03319712822 [Google Scholar] [CrossRef] [PubMed]
[3]. Ordóñez NG, Mackay B, Granular cell tumour: A review of the pathology and histogenesisUltrastruc Pathol 1999 23(4):207-22.10.1080/01913129928154510503740 [Google Scholar] [CrossRef] [PubMed]
[4]. Kim EY, Kang DK, Kim TH, Jung YS, Kim KS, Yim H, Granular cell tumour of the male breast: Two case descriptions and brief review of the literatureJ Ultrasound Med 2011 30(9):1295-301.10.7863/jum.2011.30.9.129521876101 [Google Scholar] [CrossRef] [PubMed]
[5]. Simone ND, Aggon A, Christy C, Granular cell tumour of the breast: Clinical and pathologic characteristics of a rare case in a 14-year old girlJ Clin Oncol 2011 29(22):e656-e57.10.1200/JCO.2011.35.944821646617 [Google Scholar] [CrossRef] [PubMed]
[6]. Adeniran A, Al-Ahmadie H, Mahoney MC, Robinson-Smith TM, Granular cell tumour of the breast: A series of 17 cases and review of the literatureBreast J 2004 10(6):528-31.10.1111/j.1075-122X.2004.21525.x15569210 [Google Scholar] [CrossRef] [PubMed]
[7]. Lack EE, Worsham GF, Callihan MD, Crawford BE, Klappenbach S, Rowden G, Granular cell tumour: A clinicopathologic study of 110 patientsJ Surg Oncol 1980 13(4):301-16.10.1002/jso.29301304056246310 [Google Scholar] [CrossRef] [PubMed]
[8]. Papalas JA, Wylie JD, Dash RC, Recurrence risk and margin status in granular cell tumours of the breast: A clinicopathologic study of 13 patientsArch Pathol Lab Med 2011 135(7):890-95. [Google Scholar]
[9]. Epstein DS, Pashaei S, Hunt E Jr, Fitzpatrick JE, Golitz LE, Pustulo-ovoid bodies of Milian in granular cell tumoursJ Cutan Pathol 2007 34(5):405-09.10.1111/j.1600-0560.2006.00632.x17448196 [Google Scholar] [CrossRef] [PubMed]
[10]. Le BH, Boyer PJ, Lewis JE, Kapadia SB, Granular cell tumour, immunohistochemical assessment of inhibin-alpha protein gene product 9,5,S100 protein, CD68 and ki67 proliferase index with clinical correctionArch Pathol Lab Med 2004 128(7):771-75. [Google Scholar]
[11]. Lester SC, Hicks DG, Diagnostic Pathology: Breast 2016 2nd edSalt Lake City, UtahAmirsys ElsevierISSN 9780323377126 [Google Scholar]
[12]. Willén R, Willén H, Balldin G, Albrechtsson U, Granular cell tumour of the mammary gland simulating malignancy. A report on two cases with light microscopy, transmission electron microscopy and immunohistochemical investigationVirchows Arch A Pathol Anat Histopathol 1984 403(4):391-400.10.1007/BF007372886330972 [Google Scholar] [CrossRef] [PubMed]