Pleural Mesothelioma Diagnosed on Inguinal Lymph Node Biopsy: A Rare Entity
Divya Singh1, Rekha Bhandari2, Surender Bana3, Deepak Sundriyal4, Arvind Kumar Gupta5
1 Senior Resident, Department of Pathology and Lab Medicine, AIIMS, Rishikesh, Uttarakhand, India.
2 Senior Resident, Department of Pathology and Lab Medicine, AIIMS, Rishikesh, Uttarakhand, India.
3 Senior Resident, Department of Radiodiagnosis, AIIMS, Rishikesh, Uttarakhand, India.
4 Assistant Professor, Department of Medical Oncology, AIIMS, Rishikesh, Uttarakhand, India.
5 Associate Professor, Department of Pathology and Lab Medicine, AIIMS, Rishikesh, Uttarakhand, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Arvind Kumar Gupta, Department of Pathology, AIIMS, Rishikesh, Uttarakhand, India.
E-mail: arvindgupta.path@gmail.com
Mesothelioma is a locally aggressive tumour arising from mesothelial lining of serous cavities, commonest site being the pleura. Distant metastasis to extra thoracic lymph node is rare. We herewith report a case of pleural mesothelioma with extensive peritoneal involvement which was diagnosed after inguinal node biopsy. This case highlights the aggressive nature of mesothelioma along with unusual site of metastasis. Though rare, a differential diagnosis of malignant mesothelioma should be kept while dealing with patients presenting with ascites and inguinal lymphadenopathy. A high index of suspicion along with immunohistochemical confirmation can prevent disease progression and prolong patient survival.
Asbestosis, Inguinal lymphadenopathy, Malignant pleural mesothelioma
Case Report
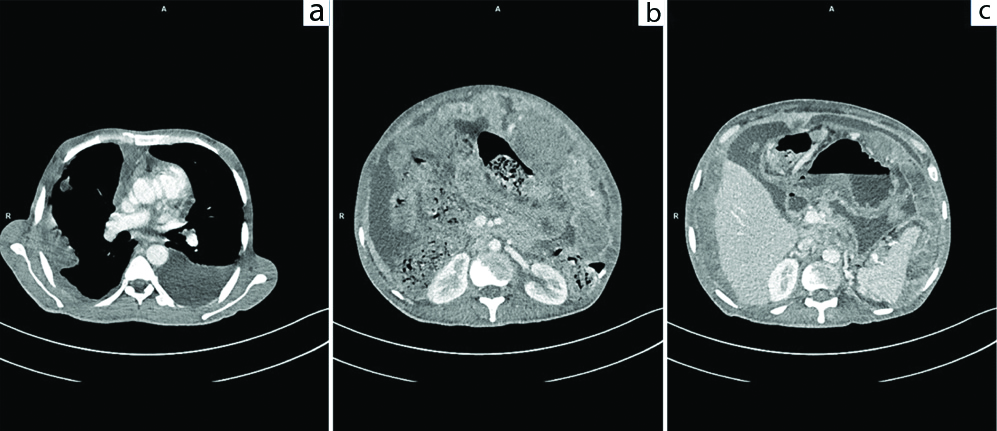
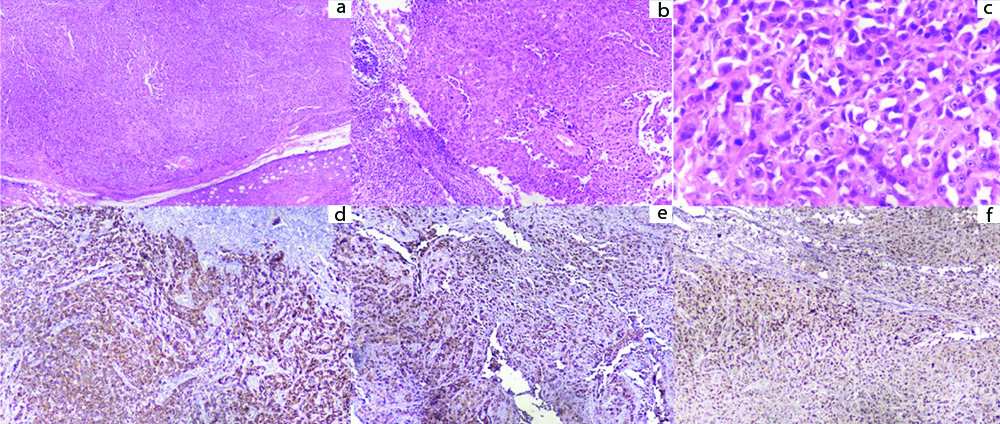
A 42-year-old male patient present with dyspnoea, ascites and loss of appetite for eight months. He had been evaluated extensively in multiple hospitals outside but a definite diagnosis could not be made. He was started with anti-tubercular therapy since past six months empirically but his condition did not improve. Even PET CT and peritoneal biopsy done outside were inconclusive. In our institute, he was evaluated thoroughly and on examination gross ascites and inguinal lymphadenopathy was present. Ascitic fluid showed high Serum Ascites Albumin Gradient (SAAG-1.7 g/dL), low Adenosine deaminase level along with raised serumLDH levels (2830 U/L). Blood examination showed Hb -13.4 g/dL, Total Leucocyte Count -16800 cells/cubic millimetre with Neutrophils-91%, Lymphocytes-5% and Platelet-4.07 Lacs/cubic millimeter. Cytological examination of ascitic fluid was done thrice but it failed to reveal the presence of any malignant cells. CECT abdomen showed extensive deposits in the omentum forming omental caking along the peritoneum with contiguous right pleuro-peritoneal spread, right pleural deposits, hilar lymphadenopathy and colonic thickening suspecting a possibility of caecal/appendicular mucinous adenocarcinoma [Table/Fig-1a-c]. Here patient refused to undergo pleural biopsy, hence inguinal node biopsy was done. Histopathological examination of inguinal node revealed complete effacement of nodal architecture by a high grade neoplasm comprising of sheets and nests of pleomorphic tumour cells having prominent nucleoli and moderate amount of pale eosinophilic cytoplasm [Table/Fig-2a-c]. Differentiation could not be made on morphology alone hence immunohistochemistry was performed, keeping a few differentials in mind- poorly differentiated squamous cell carcinoma, poorly differentiated adenocarcinoma, amelanotic melanoma and malignant mesothelioma. The tumour cells came positive for PANCK, calretinin and WT1 and negative for p40, MOC-31, TTF-1, CK7, CK20, HMB45 and melan A [Table/Fig-2d-f]. Based on these findings a diagnosis of malignant mesothelioma was given. The patient was planned for chemotherapy but within 24 hours the patient developed sudden cardiac arrest and succumbed to death.
a) CECT Thorax axial image showing bilateral pleural effusion (left>right) with enhancing pleural based deposits on right side. b,c) CECT abdomen and pelvis axial images showing extensive omental soft tissue deposits forming omental caking, enhancing nodular peritoneal thickening, deposits along serosal surface of bowel and gross ascites.
a) Low power view showing solid sheets of tumour cells (H&E, X40). b) Low power view showing solid sheets of tumour cells with few blood vessels (H&E, X100); c) Tumour cells are markedly pleomorphic having hyperchromatic nucleus with prominent nucleoli and moderate amount of cytoplasm (H&E, X400); d,e,f) Immunohistochemistry revealed tumour cells positive for cytokeratin, calretinin and WT1 respectively (Immunoperoxidase, X400).
Discussion
Mesothelioma is a locally aggressive tumour arising from mesothelial lining of serous cavities, commonest site being the pleura [1]. Distant metastasis to extra thoracic lymph node is rare and it is even more unusual to find the initial clinical presentation as systemic lymph node metastasis. Zhang Y et al., described three cases of malignant mesotheliomas with systemic lymphadenopathy as the initial presentation [2]. Malignant mesothelioma account for approximately 0.2% of all malignant tumours, and cases presenting with distant metastasis is extremely rare [3]. Making diagnosis at an early stage of the disease is usually difficult due to rarity of the lesion and vagueness of symptoms at the time of presentation, as we also saw in the present case because of multiorgan involvement. The causation has been directly linked to asbestos exposure but we did not find any history of asbestos exposure in the present case.
The common sites of metastasis are liver, bone, brain, adrenal gland, kidney, pancreas, thyroid, spleen, skin and lymph nodes, mostly reported as autopsy findings. Patients presenting with distant lymph node involvement is very rare [4]. Histologically, three variants of mesothelioma are described-epithelioid, sarcomatoid or mixed [5].
The common subtypes of epithelioid mesotheliomas are further subtyped on morphology as: tubulopapillary, acinar, adenomatoid, solid and rare ones including clear, deciduoid, signet ring and small cell variant [6]. This further needs immunohistochemical confirmation by the use of panel of antibodies including at least two mesothelial markers and two carcinoma markers depending upon the differential diagnosis under consideration along with use of cytokeratin. Staining in 10% of tumour cells has been considered positive for supporting the diagnosis. Molecular studies demonstrating deletions of p16/CDKN2A is known to occur in malignant mesotheliomas and help to differentiate from benign mesothelial proliferation [7].
It is uncommon for patients to have associated ascites or peritoneal involvement from a primary pleural tumour whereas those with diffuse peritoneal involvement commonly present with ascites. In the present case both pleural, peritoneal as well as caecal involvement was present. Both the pleural and peritoneal mesotheliomas have a low propensity for distant metastasis but regional spread is common. Metastasis of malignant mesothelioma to the pleural cavity and chest wall is common and can also spread to supraclavicular and axillary lymph node [8]. Cases of malignant mesothelioma with metastatic lymphadenopathy are described in [Table/Fig-3] [2-4,9-11].
Cases of malignant mesothelioma with Metastatic lymphadenopathy.
| Studies | Cases | Cases of Mesothelioma with subsequent metastasis |
|---|
| Zhang Y et al., [2] | Three | Malignant Mesothelioma metastasized to Systemic Lymphadenopathy |
| Takehara Y et al., [3] | One | Malignant peritoneal mesothelioma metastasized to lymph node metastasis within the transverse colon |
| Kant S et al., [4] | One | Malignant pleural mesothelioma metastasized to a peripheral lymph node |
| Raju BVLN et al., [9] | One | Pleural Mesothelioma metastasized to Inguinal Lymph node |
| Sussman J and Rosai J [10] | Six | Malignant mesothelioma metastasized to Lymph node- cervical in four cases, mediastinal in one, and inguinal in one |
| King JA, et al., [11] | One | Malignant mesothelioma metastasized to inguinal lymph node |
| Present case | One | Malignant mesothelioma metastasized to inguinal lymph node metastases |
Histomorphology associated with poor prognosis include nonepithelioid subtypes morphology especially the desmoplastic-variant sarcomatoid mesothelioma [12]. The pleomorphic-variant phenotype has a poor prognosis [13]. Nuclear grading (degree of nuclear atypia and mitotic count and/or MIB-1 labeling index) has been shown to be a strong predictor of overall survival in diffuse pleural and peritoneal mesothelioma [14].
Conclusion(s)
Hereby a case of pleural mesothelioma with extensive peritoneal involvement which was diagnosed after inguinal node biopsy has been reported. This case highlights the aggressive nature of mesothelioma along with unusual site of metastasis. Though rare, a differential diagnosis of malignant mesothelioma should be kept while dealing with patients presenting with ascites and inguinal lymphadenopathy. A high index of suspicion along with immunohistochemical confirmation can prevent disease progression and prolong patient survival.
Declaration: Patient died due to sudden cardiac arrest and family member could not be contacted for informed consent. Authors have taken utmost care to de-identify the patient’s details.
Author Declaration:
Financial or Other Competing Interests: No
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. No
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Nov 01, 2019
Manual Googling: Nov 28, 2019
iThenticate Software: Dec 26, 2019 (16%)
[1]. Ahmed I, Koulaouzidis A, Iqbal J, Tan WC, Malignant peritoneal mesothelioma as a rare cause of ascites: A case reportJournal of Medical Case Reports 2008 2:12110.1186/1752-1947-2-12118439258 [Google Scholar] [CrossRef] [PubMed]
[2]. Zhang Y, Taheri ZM, Jorda M, Systemic lymphadenopathy as the initial presentation of malignant mesothelioma. A report of three cases:Patholog Res Int 2010 2010:84657110.4061/2010/84657121151527 [Google Scholar] [CrossRef] [PubMed]
[3]. Takehara Y, Endo S, Mori Y, Nakahara K, Takayanagi D, Shimada S, Malignant peritoneal mesothelioma with lymph node metastasis that originated in the transverse colonWorld J Surg Oncol 2014 12:11210.1186/1477-7819-12-11224754918 [Google Scholar] [CrossRef] [PubMed]
[4]. Kant S, Verma SK, Sanjay Malignant pleural mesothelioma without asbestos exposure with distant metastasis in a peripheral lymph node: A case reportLung India 2008 25(1):31-33.10.4103/0970-2113.4413720396658 [Google Scholar] [CrossRef] [PubMed]
[5]. Barreiro TJ, Katzman PJ, Malignant mesothelioma: A case presentation and reviewThe Journal of American Osteopathic Association 2006 106(12):699-704. [Google Scholar]
[6]. Brcic L, Vlacic G, Quehenberger F, Kern I, Reproducibility of malignant pleural mesothelioma histopathologic subtypingArch Pathol Lab Med 2018 142(6):747-52.10.5858/arpa.2017-0295-OA29509030 [Google Scholar] [CrossRef] [PubMed]
[7]. Husain AN, Colby TV, Ordóñez NG, Allen TC, Attanoos RL, Beasley MB, Guidelines for pathologic diagnosis of malignant mesothelioma 2017 Update of the consensus statement from the international mesothelioma interest groupArch Pathol Lab Med 2018 142(1):89-108.10.5858/arpa.2017-0124-RA28686500 [Google Scholar] [CrossRef] [PubMed]
[8]. Kubota K, Furuse K, Kawahara M, Ogawara M, Ryu S, Yamamoto S, A case of malignant pleural mesothelioma with metastasis to the orbitJpn J Clin Oncol 1996 26(6):469-71.10.1093/oxfordjournals.jjco.a0232669001354 [Google Scholar] [CrossRef] [PubMed]
[9]. Raju BVLN, Kotilingam K, Babu GR, Ramakoteswarao N, Pleural mesothelioma with inguinal lymphnode metastasis- A case reportLung India 1989 7(1):44-47. [Google Scholar]
[10]. Sussman J, Rosai J, Lymph node metastasis as the initial manifestation of malignant mesothelioma. Report of six casesAm J Surg Pathol 1990 14(9):819-28.10.1097/00000478-199009000-000032389813 [Google Scholar] [CrossRef] [PubMed]
[11]. King JA, Listinsky CM, Tucker JA, An intriguing case: Malignant mesothelioma presenting as inguinal lymph node metastasesUltrastruct Pathol 2004 28(2):109-13.10.1080/0191312049043099515205111 [Google Scholar] [CrossRef] [PubMed]
[12]. Curran D, Sahmoud T, Therasse P, van Meerbeeck J, Postmus PE, Giaccone G, Prognostic factors in patients with pleural mesothelioma: The European Organization for Research and Treatment of Cancer experienceJ ClinOncol 1998 16(1):145-52.10.1200/JCO.1998.16.1.1459440736 [Google Scholar] [CrossRef] [PubMed]
[13]. Ordóñez NG, Pleomorphic mesothelioma: Report of 10 casesMod Pathol 2012 25(7):1011-22.10.1038/modpathol.2012.3922388762 [Google Scholar] [CrossRef] [PubMed]
[14]. Musk AW, Olsen N, Alfonso H, Reid A, Mina R, Franklin P, Predicting survival in malignant mesotheliomaEur Respir J 2011 38(6):1420-24.10.1183/09031936.0000081121737558 [Google Scholar] [CrossRef] [PubMed]