Study to Evaluate the Role of Inflammatory Biomarker- IL-6 in Obstructive Sleep Apnea in Correlation with Apnea-Hypopnea Index
Deepanjali Sharma1, Deepak Kumar Shah2, Anju Bharti3, J K Mishra4
1 Junior Resident, Department of T.B. and Respiratory Diseases, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
2 Assistant Professor, Department of T.B. and Respiratory Diseases, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
3 Assistant Professor, Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
4 Professor, Department of T.B. and Respiratory Diseases, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Anju Bharti, Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, Uttar Pradesh, India.
E-mail: anjubhartimeena@gmail.com
Introduction
Many biomarkers are associated with the Apnea-Hypopnea Index (AHI) in the majority of the studies, but most of the explored approaches were not able to identify definitive biomarkers of Obstructive Sleep Apnea (OSA) morbidity.
Aim
To evaluate the correlation of AHI with inflammatory biomarker-Interleukin-6 (IL-6).
Materials and Methods
Adult patients above 18 years of age, and suffering from OSA or had history suggestive of OSA were included in the study. IL-6 was measured by ELISA kit and immunoassay equipment. Polysomnography (PSG) study was done which included sleep stages, Heart rhythm monitoring, Leg movements recording, Breathing monitoring. Obstructive apnea was taken as cessation of airflow for at least 10 seconds with persistent respiratory effort. Descriptive statistical methods were used such as computing percentage, mean, standard deviation, pivot tables, and correlation.
Results
A total number of 46 patients with OSA were evaluated. Out of 46 patients, 9 (19.57%), 6 (13.04%) and 31 (67.39%) patients belonged into mild, moderate and severe AHI, respectively. ELISA for IL-6 was done in 41 patients. The value ranged from 20.92 to 4145.23 pg/mL with SD±1769.32 and with mean of 1427.94. Average values of biomarker IL-6 was 675.26 pg/mL, 1261.19 pg/mL, 1658.58 pg/mL in mild, moderate and severe OSA respectively. There was a positive correlation between AHI severity and IL-6 with correlation value.
Conclusion
In this study, IL-6 appeared to exhibit a favourable profile for a biomarker aiming to discriminate OSA in adult patients. However more large scale studies are required to establish a definitive correlation between OSA and IL-6.
Enzyme linked immunosorbent assay, Inflammation, Polysomnography
Introduction
An OSA is by far the most common sleep disorder and estimated to occur in 34% of men and 17% of women, afflicting more than 100 million adults worldwide [1-3]. It involves a reduce or complete halt in airflow due to collapse of upper airways during sleep. Moreover, OSA is a well-known risk factor for work related and motor vehicle accidents and when left untreated, it has been associated with increased health morbidity, and mood deficits that reduces the work efficiency and productivity. Consequently, healthcare costs are substantially increased for patients with OSA, accounting either directly or via its associated morbidities for a considerable proportion of all medical related expenses. To prevent the health consequences of OSA, early identification and optimal treatment of OSA is necessary. Currently, the role of co-morbidities in OSA is a major topic in clinical research as is shown by the rising number of publications on this subject [4,5]. Several recent epidemiologic studies reported a high prevalence of co-morbidities in OSA, some more prevalent than the others [6-9]. The severity of OSA is most often reported in terms of AHI. A patient diagnosed with OSA should have AHI more than five episodes per hour of cessation of breathing for at least 10 seconds on overnight PSG. The severity of sleep apnoea is categorised as Mild OSAS: 5<AHI<15, Moderate OSAS: 15<AHI<30 and Severe OSAS: AHI >30. Oxygen desaturation accompanying apneic events, negative intrathoracic pressure, arousals induced by upper airway obstruction and repeated activation of the sympathetic system may cause abnormal activation of neural, hormonal, thrombotic, metabolic, and inflammatory responses [10,11]. The potential aetiological mechanisms may include endothelial dysfunction, oxidative stress, inflammation, and disorders of coagulation and lipid metabolism [12]. Throughout the most recent 14 years, a few diverse OSA biomarkers have been proposed. Despite the fact that the legitimacy of biomarkers in the determination of OSA stays vague, however in grown-ups IL-6 appears to have a positive potential to turn into a decent biomarker to distinguish OSA [13]. So, present study was conducted to evaluate the correlation of AHI with Inflammatory biomarker- IL-6.
Materials and Methods
Present cross-sectional study was carried out from 1st October 2017 to 31st March 2019 (18 Months) at the Department of Respiratory Medicine in collaboration of Department of Pathology at Institute of Medical Sciences, Banaras Hindu University, after getting approval from the ethical committee (IEC- Dean/2017/EC/204).
Inclusion Criteria
Patients willing and able to participate in the study, adult patients above 18 years of age, and suffering from OSA or had history suggestive of OSA were included in the study.
Exclusion Criteria
Unstable medical or psychiatric conditions that would interfere in performing the study. Chronic respiratory failure or insufficiency, recent myocardial infarction, exacerbation of obstructive airway diseases, Patients having a history of upper airway surgery, disorders of a craniofacial abnormality like Marfan’s syndrome, Down’s syndrome, Pierre-Robin syndrome.
Detailed history and physical examination with special attention to risk factors of OSA was done. Patients’ height, weight, neck circumference, neck length, abdominal and waist circumference were measured. Body Mass Index (BMI)- BMI >30 kg/m2 was considered as obese. Neck Circumference (NC) >37 cm in men and >34 cm in women was set as the upper limit of overweight/obesity. Neck Length (NL) or Mentohyoid distance of less than 3 finger-width was considered as a strong association with OSA. Abdominal Circumference (AC) and Waist Circumference (WC): AC in Men: >102 cm; in Women: >88 cm taken as central obesity. WC Men: ≥90 cm; women: ≥80 cm and Waist Height Ratio (WHtR) 0.5 was taken as a cut-off for central obesity. Blood tests- Haematocrit, Glucose Fasting and Post-prandial, Lipid Profile- cholesterol, triglycerides, DHL, LDL, VLDL Thyrotropin (TSH), ELISA for IL-6 Normal range of haematocrit - 45% to 52% for men and 37% to 48% for women. Patients with blood glucose ≥110 mg/dL were considered diabetic. Patients with normal sugar values on medication were taken as a diabetic patient. Patients with triglycerides ≥150 mg/dL and HDL cholesterol <40 mg/dL in men and <50 mg/dL in ladies were delegated patient of dyslipidaemia. Diagnosis of metabolic syndrome was made in the presence of at least 3 of the 5 significant clinical signs i.e., Obesity, Hypertension, Diabetes mellitus, Hypertriglyceridaemia, Hypercholesterolaemia. IL-6 was measured by ELISA kit (Immunoshop India Pvt., Ltd.,) and immunoassay equipment. PSG study was done which included sleep stages, Heart rhythm monitoring, Leg movements recording, Breathing monitoring. Obstructive apnea was taken as cessation of airflow for at least 10 seconds with persistent respiratory effort. Following parameter were noted- AHI - Categorised as Mild OSAS: 5<AHI<15, Moderate OSAS: 15<AHI<30 and Severe OSAS: AHI >30.
Statistical Analysis
Data collected for the sample was analysed using Microsoft Excel 2016. In present study, we used descriptive statistical methods such as computing percentage, mean, standard deviation, pivot tables, and correlation. SPSS Inc. (233 South Wacker Drive, 11th Floor Chicago, IL 60606-6412) software was used and appropriate tests were employed for categorical, parametric or non-parametric data. Wherever necessary, the results of the study are depicted in the form of charts, tables, graphs and percentages.
Results
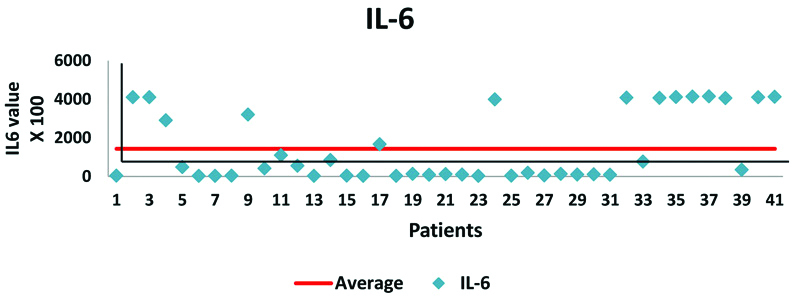
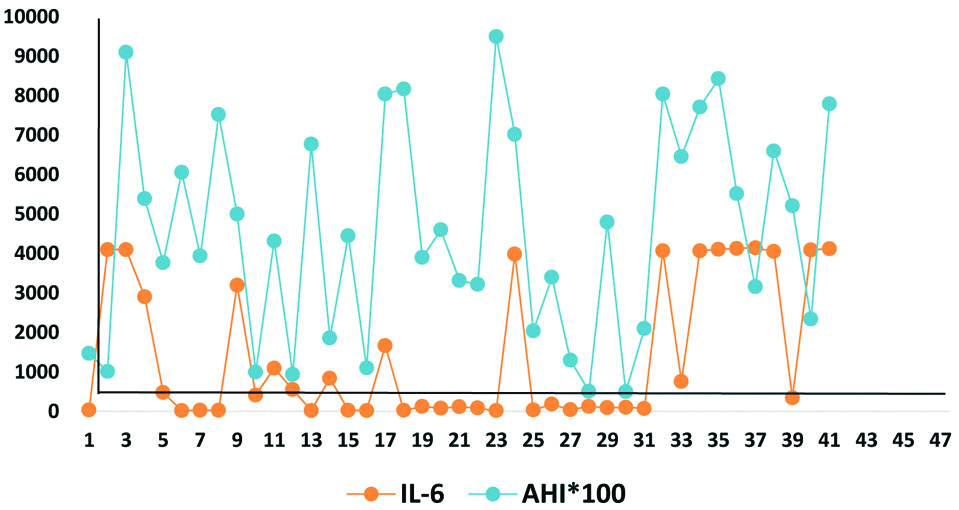
A total number of 46 patients with OSA were evaluated, out of which 21 (46%) were females and 25 (54%) were males. Maximum number of male 7 (15%) and female 10 (22%) patients belonged to age group 50-59 and 60-69 years, respectively [Table/Fig-1]. The study showed that the average age of female and male patients was almost similar (58.8 yrs. Vs. 58 yrs.). In the study, 24% of males were smokers followed by the history of alcohol intake and tobacco chewing in 8% each. No female patient was having a history of smoking or tobacco chewing but 38% of females was exposed to biomass/kerosene fuel smoke at home. The severity of OSA was divided into Mild, Moderate and Severe AHI according to the number of apnoea episodes/hr. on Polysomnography (PSG) study. Mild, Moderate and Severe AHI is shown in [Table/Fig-2]. Due to financial issue, ELISA for IL-6 (Inflammatory Biomarker) was done in 41 patients only. The value ranged from 20.92 to 4145.23 pg/mL with SD±1769.32 pg/mL and Mean of 1427.94 pg/mL [Table/Fig-3,4]. Average values of biomarker IL-6 was estimated 675.26 pg/mL in mild OSA, 1261.19 pg/mL in Moderate ODA and 1658.58 pg/mL in severe OSA. There was a Positive correlation between AHI severity and IL-6 with Correlation Value (CV) of +0.369867430051592, means if the severity of OSA increases the IL-6 value will also increase and vice versa. It is also represented in [Table/Fig-5].
Age-Sex distribution of patients with OSA.
| Sex | Age (years) |
|---|
| 30-39 | 40-49 | 50-59 | 60-69 | 70-79 | 80-89 | Total % | Total No. |
|---|
| % | No | % | No | % | No | % | No | % | No | % | No | | |
|---|
| Female | 2% | 1 | 2% | 1 | 20% | 9 | 22% | 10 | 0% | 0 | 0% | 0 | 46% | 21 |
| Male | 4% | 2 | 11% | 5 | 15% | 7 | 11% | 5 | 7% | 3 | 7% | 3 | 54% | 25 |
| Grand total | 7% | 3 | 13% | 6 | 35% | 16 | 33% | 15 | 7% | 3 | 7% | 3 | 100% | 46 |
Severity of OSA and mean, mode and standard deviation of AHI.
| AHI Grade |
|---|
| Mild (5<AHI<15) | 9 | 19.57% |
| Moderate (15<AHI<30) | 6 | 13.04% |
| Severe (AHI>30) | 31 | 67.39% |
| Grand total | 46 | 100% |
| AHI |
| Mean | 44.3 |
| Median | 41.3 |
| Mode | 80.4 |
| Standard deviation | 26.9 |
| Range | 90 |
| Minimum | 5 |
| Maximum | 95 |
Showing mean, mode, median and standard deviation in 41 patients with OSA.
| IL-6 (Pg./mL) |
|---|
| Mean | 1427.94 |
| Median | 341.6666 |
| Mode | 21.87254 |
| Standard Deviation | 1769.326 |
| Minimum | 20.9292 |
| Maximum | 4145.235 |
| Sum | 58545.56 |
| Count | 41 |
Shows IL-6 value in 41 patients with OSA (AHI value scaled by 100).
Shows positive correlation value between AHI and IL-6.
(*IL-6 values shown are multiplied by 100)
Discussion
OSA is characterised by a recurrent partial or complete collapse of upper airways during sleep. In a survey from northern part of India, the prevalence of OSA Syndrome is estimated as 3.6% and separately for males and females it was 4.9% and 2.1%, respectively [14]. In this study, we included 21 (46%) female and 25 (54%) male patients who clinically had symptoms suggestive of OSA and underwent PSG to access severity of AHI. Present study showed that the average age of female and male patients was almost similar (58.8 yrs vs. 58 yrs), which was higher as compared to other study. The sex distribution of the patients was similar in the both studies [15].
Patients with OSAS are always exposed to intermittent hypoxia and re-oxygenation from the cycles of apnoea/arousals. It is suggested that sustained hypoxia leads to oxidative stress and activation of a systemic inflammatory response with increase in general blood antioxidant activity and in production of pro-inflammatory cytokines, including Tumour Necrosis Factor TNF-α and interleukin-6 [16]. In the present study, we had selected IL-6 as a biomarker to evaluate its levels in 41 patients with OSA. We correlated IL-6 levels with AHI. The average value of IL-6 was 1427.94 pg/mL, ranging from 20.9292 to 4145.235 pg/mL. Average values of IL-6 were estimated by Immunoassay (ELISA) as 675.26 pg/mL in mild OSA, 1261.19 pg/ml in Moderate OSA and 1658.58 pg/mL in severe OSA. A study was conducted by Podkówka R et al., to estimate IL-6 levels in 29 healthy young adults (27.0±4.2 years) and 30 healthy elderly (71.1±5.5 years). The level of interleukin-6 was five times higher in the patient’s who belonged to the older age group, but it was not statistically significant [17]. The findings in present study are also in support of some other studies [18-22]. PSG is a poor predictor of OSA-associated morbidities. Two patients with similar OSA severity may behave with markedly different clinical phenotypes. That’s why such studies have importance to enable incorporation of morbidity biomarkers into well-defined and validated clinical guidelines or algorithms [23].
The searching out of an important biomarker for OSA associated morbidity has the potential to provide knowledge related to prognosis and response to therapy. Blood-based biomarkers were accounted in the majority of the studies, and most of the explored approaches did not identify definitive biomarkers of OSA morbidity. Studies showed that interleukin-6 can be considered as a biomarker to differentiate the patients with OSA with different co-morbidities [24]. Furthermore, in the present study, the average value of IL-6 was significantly higher than general young and elderly population thus, we can presume that high levels were because of OSA but, cannot confirm that these levels were because of OSA or associated comorbidities, or both.
Limitation(s)
First limitation is a small sample size, and the second is that we did not rely on a control group, comprising individuals without OSA, to establish correlation with the associated morbidities.
Conclusion(s)
Blood-based biomarkers accounted for the majority of the studies, but most of the studies did not identify a reliable biomarker of OSA morbidity.
In present study IL-6 levels have shown positive correlation with AHI. However, more large scale studies are required to establish a definitive correlation between OSA and IL-6. These findings warrant further research with case-control studies and standard statistical analyses to have a better understanding of interrelationships of these cytokines.
Author Declaration:
Financial or Other Competing Interests: No
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Oct 17, 2019
Manual Googling: Dec 18, 2019
iThenticate Software: Dec 30, 2019 (16%)
[1]. Peppard PE, Young T, Palta M, Skatrud J, Prospective study of the association between sleep-disordered breathing and hypertensionN Engl J Med 2000 342(19):1378-84.10.1056/NEJM20000511342190110805822 [Google Scholar] [CrossRef] [PubMed]
[2]. Watson NF, Health care savings: the economic value of diagnostic and therapeutic care for obstructive sleep apneaJ Clin Sleep Med 2016 12(8):1075-77.10.5664/jcsm.603427448424 [Google Scholar] [CrossRef] [PubMed]
[3]. Bousquet J, Khaltaev N, World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach 2007 GenevaWHOAvailable from: www.who.int/gard/publications/GARD [Google Scholar]
[4]. Wolk R, Shamsuzzaman AS, Somers VK, Obesity, sleep apnea, and hypertensionHypertension 2003 42(6):1067-74.10.1161/01.HYP.0000101686.98973.A314610096 [Google Scholar] [CrossRef] [PubMed]
[5]. Bonsignore MR, Baiamonte P, Mazzuca E, Castrogiovanni A, Marrone O, Obstructive sleep apnea and comorbidities: a dangerous liaisonMultidisciplinary Respiratory Medicine 2019 14(1):810.1186/s40248-019-0172-930809382 [Google Scholar] [CrossRef] [PubMed]
[6]. Mokhlesi B, Ham SA, Gozal D, The effect of sex and age on the comorbidity burden of OSA: An observational analysis from a large nationwide US health claims databaseEur Respir J 2016 47(4):1162-69.10.1183/13993003.01618-201526797029 [Google Scholar] [CrossRef] [PubMed]
[7]. Senaratna CV, English DR, Currier D, Perret JL, Lowe A, Lodge C, Sleep apnoea in Australian men: Disease burden, co-morbidities, and correlates from the Australian longitudinal study on male healthBMC Public Health 2016 16(Suppl 3):102910.1186/s12889-016-3703-828185594 [Google Scholar] [CrossRef] [PubMed]
[8]. Appleton SL, Gill TK, Lang CJ, Taylor AW, McEvoy RD, Stocks NP, Prevalence and comorbidity of sleep conditions in Australian adults: 2016 Sleep Health Foundation national surveySleep Health 2018 4(1):13-19.10.1016/j.sleh.2017.10.00629332673 [Google Scholar] [CrossRef] [PubMed]
[9]. Tveit RL, Lehmann S, Bjorvatn B, Prevalence of several somatic diseases depends on the presence and severity of obstructive sleep apneaPLoS ONE 2018 13(2):e019267110.1371/journal.pone.019267129474482 [Google Scholar] [CrossRef] [PubMed]
[10]. Shamsuzzaman ASM, Gersh BJ, Somers VK, Obstructive sleep apnea: Implications for cardiac and vascular diseaseJAMA 2003 290(14):1906-14.10.1001/jama.290.14.190614532320 [Google Scholar] [CrossRef] [PubMed]
[11]. Eisensehr I, Ehrenberg BL, Noachtar S, Korbett K, Byrne A, McAuley A, Platelet activation, epinephrine, and blood pressure in obstructive sleep apnea syndromeNeurology 1998 51(1):188-95.10.1212/WNL.51.1.1889674801 [Google Scholar] [CrossRef] [PubMed]
[12]. Stiefel P, Sanchez-Armengol MA, Villar J, Vallejo-Vaz A, Moreno-Luna R, Capote F, Obstructive sleep apnea syndrome, vascular pathology, endothelial function and endothelial cells and circulating microparticlesArch Med Res 2013 44(6):409-14.pmid:2405104110.1016/j.arcmed.2013.08.00524051041 [Google Scholar] [CrossRef] [PubMed]
[13]. De Luca Canto G, Pachêco-Pereira C, Aydinoz S, Major PW, Flores-Mir C, Gozal D, Biomarkers associated with obstructive sleep apnea and morbidities: A scoping reviewSleep Medicine 2015 16(3):347-57.10.1016/j.sleep.2014.12.00725747333 [Google Scholar] [CrossRef] [PubMed]
[14]. Sharma SK, Kumpawat S, Banga A, Goel A, Prevalence and risk factors of obstructive sleep apnoea syndrome in a population of Delhi, IndiaChest 2006 130(1):149-56.10.1378/chest.130.1.14916840395 [Google Scholar] [CrossRef] [PubMed]
[15]. Motamedi V, Kanefsky R, Matsangas P, Mithani S, Jeromin A, Brock MS, Elevated tau and interleukin-6 concentrations in adults with obstructive sleep apneaSleep Med 2018 3(43):71-76.10.1016/j.sleep.2017.11.112129482817 [Google Scholar] [CrossRef] [PubMed]
[16]. Lurie A, Inflammation, oxidative stress, and procoagulant and thrombotic activity in adults with obstructive sleep apneaAdv Cardiol 2011 46:43-66.10.1159/00032510522005189 [Google Scholar] [CrossRef] [PubMed]
[17]. Podkówka R, Wiśniewska J, Korybalska K, Wieczorowska-Tobis K, Knapowski J, Plasma level of interleukin-6 and interleukin-8 in the elderlyPrzegl Lek 2002 59(4-5):230-33. [Google Scholar]
[18]. Thunstrom E, Glantz H, Fu M, Yucel-Lindberg T, Petzold M, Lindberg K, Increased inflammatory activity in nonobese patients with coronary artery disease and obstructive sleep apneaSleep 2015 38(3):463-71.10.5665/sleep.451025325463 [Google Scholar] [CrossRef] [PubMed]
[19]. Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, Elevated levels of C reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressureCirculation 2003 107(8):1129-34.10.1161/01.CIR.0000052627.99976.1812615790 [Google Scholar] [CrossRef] [PubMed]
[20]. Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP, Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesityJ Clin Endocrinol Metab 1997 82(5):1313-16.10.1210/jcem.82.5.39509141509 [Google Scholar] [CrossRef] [PubMed]
[21]. Arnardottir ES, Maislin G, Schwab RJ, Staley B, Benediktsdottir B, Olafsson I, The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: The Icelandic Sleep Apnea CohortSleep 2012 35(7):921-32.10.5665/sleep.195222754038 [Google Scholar] [CrossRef] [PubMed]
[22]. Yilmaz Avci A, Avci S, Lakadamyali H, Can U, Hypoxia and inflammation indicate significant differences in the severity of obstructive sleep apnea within similar apnea-hypopnea index groupsSleep Breath 2017 21(3):703-11.10.1007/s11325-017-1486-528271327 [Google Scholar] [CrossRef] [PubMed]
[23]. Kheirandish-Gozal L, What is “abnormal” in pediatric sleep?Respir Care 2010 55(10):1366-74.discussion 1374-1366 [Google Scholar]
[24]. Shih JL, Malhotra A, Could vitamins be helpful to patients with sleep apnea?Chest 2011 139(2):237-38.10.1378/chest.10-2017 [Google Scholar] [CrossRef]