Onychomycosis Due to Fusarium oxysporum in Sulumaniyah City, Iraq
Sazan Jamal Gharib1, Samir Khalaf Abdullah2
1 Lecturer, Department of Medical Laboratory, Sulumaniyah Technical Institute, Sulumaniyah Polytechnique University and Biology Department, University of Zakho, Iraq.
2 Professor, Department of Medical Laboratory Technology, Alnoor University College, Bartila, Nineva, Iraq.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Samir Khalaf Abdullah, Department of Medical Laboratory Technology, Alnoor University College, Bartila, Nineva, Iraq.
E-mail: samir.abdullah1947@gmail.com
Onychomycosis is a fungal infection of the nail plate of fingernails or toenails that is caused by dermatophytes, non-dermatophyte filamentous fungi or yeasts. An opportunistic pathogenic fungus was isolated from toenail of a 38-year-old female with newly diagnosed Acute Myeloid Leukaemia (AML) which appeared to belong to the genus Fusarium. Identification of aetiologic agent was performed by morphological and cultural characteristics and was confirmed by sequencing of the Internal Transcribed Spacer (ITS) region of rDNA. The isolated Fusarium oxysporum in this paper is the first case of its kind to be sequenced and reported by molecular method in Iraq.
Fungal infection, Molecular sequencing, Nail infection
Case Report
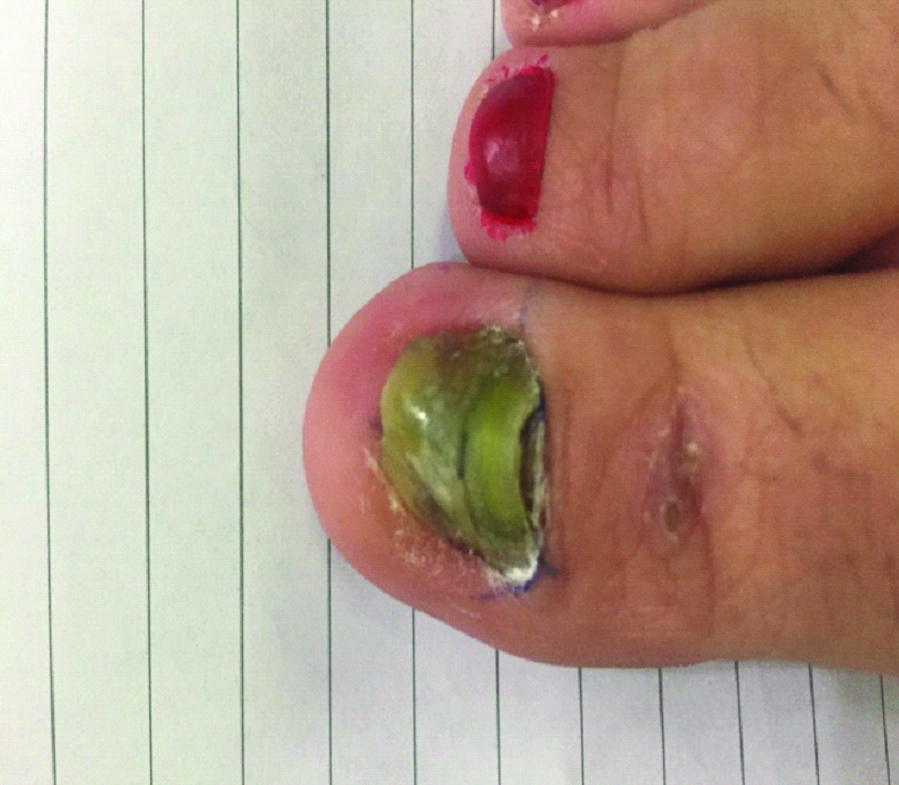
A 38-year-old female, was referred to the Dermatology out-patient clinic of Hiwa hospital (Sulumaniyah city, Iraq) in October 2016. That was the first visit of the patient to the clinic. She did not give any previous history for her case. On examination, right first toenail was dark yellowish-green to brown in colour with loss of texture and there were dystrophic changes in the nail plate of first toenail [Table/Fig-1]. The associated factor in this case was newly diagnosed acute myelogenous leukaemia for 10 months duration, and at the time of sampling, the patient had undergone chemotherapy. At day 1, oral itraconazole (200 mg/day) for two consecutive months was recommended by physician at Hiwa hospital (Sulumaniyah city, Iraq). Unfortunately, the patient did not visit the hospital; hence further management was not possible.
Dystrophic changes in the nail plate and extensive yellowish-green discolouration of large first toenail.
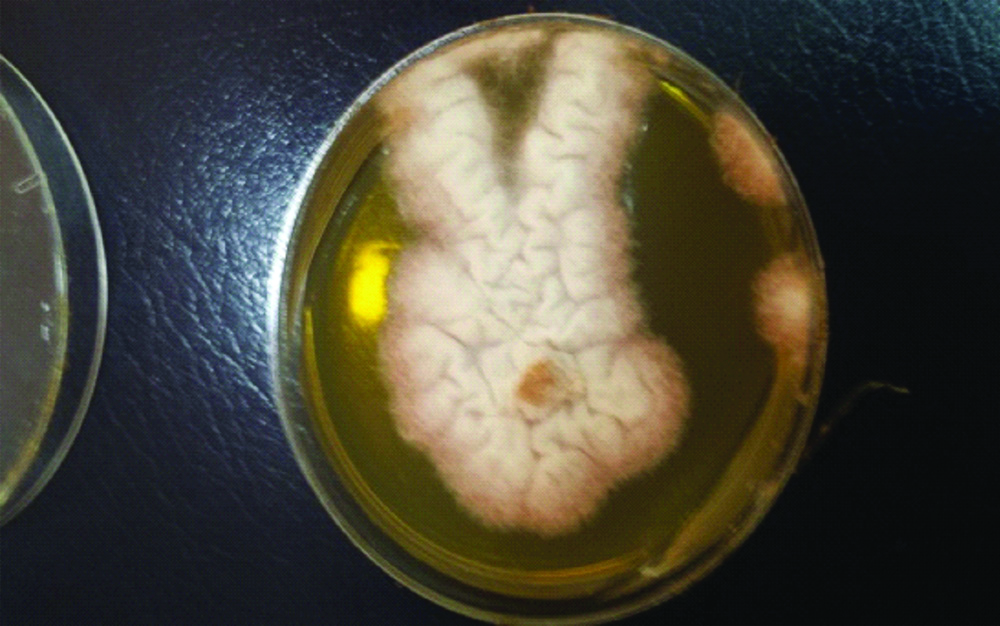
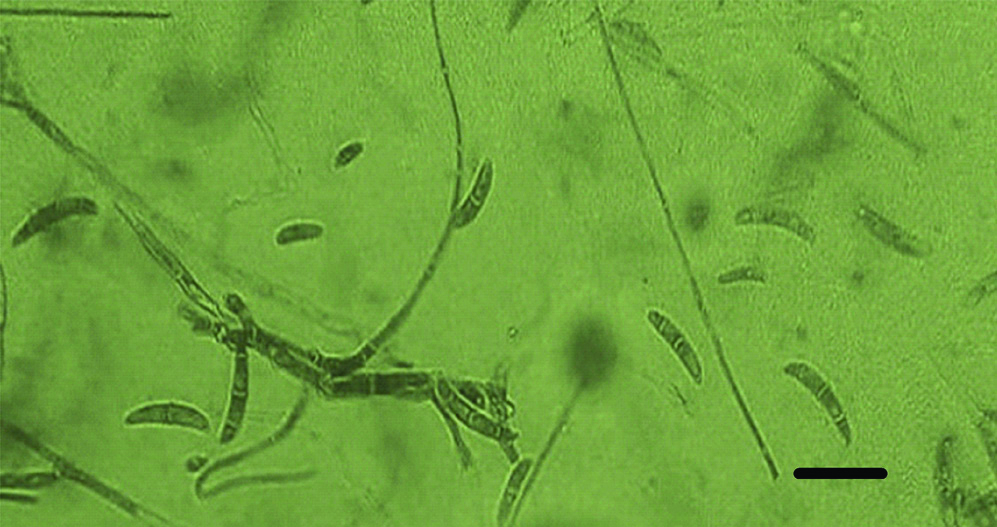
Nail clippings from the affected right great toenail was collected after proper sterilisation of the affected area with 70% alcohol. The sample was divided into two portions; one portion was observed through direct microscopic examination by mounting in 1-2 drops of 20% potassium hydroxide (KOH) solution (Merck, Germany) for 15-30 minutes, gently heated, then viewed under the light microscope (CX 40 Olympus USA) to look for fungal structures (hyaline hyphae, macro and/or micro conidia and chlamydospore) [1]. Remaining portion of the nail clippings was cultured on two sets of Sabouraud’s Dextrose Agar (SDA) medium (CM41; Oxoid, UK): two replicates of SDA plates with antibiotics chloramphenicol (SDI) (250 mg/L), and cycloheximide) (Sigma-Aldrich) (500 mg/L), and one slant of SDA contained antibiotics but without cycloheximide, which were incubated at 30°C and examined after seven days for fungal growth. The mycological test of the case, which included direct KOH examination before specimen culture was considered positive for the right first toenail. This was evident by presence of numerous hyaline branched and septate hyphae and reproductive structures suggestive of chlamydospores. Microscopic mounts were made in Lactophenol Cotton Blue (LPCB) from the white, cottony culture which later turned pink in colour examined after 10 days incubation on SDA at 30°C. The causative agent was identified as Fusarium oxysporum according to The Fusarium Laboratory Manual [Table/Fig-2,3] [2].
Fusarium oxysporum: white colony turned pink in colour on SDA medium.
LPCB wet mounts showing slightly curved hyaline septate macroconidia and oval or kidney shaped microconidia. Bar =10 um.
The method for genomic DNA extraction of the isolated F. oxysporum strain was performed as described by Gharib SJ et al., [3]. Genomic DNA was extracted and purified by taking a proper amount from fresh colony (21-day-old, grown on SDA dishes) by grinding in presence of liquid nitrogen for initial breaking up of mycelia. Total DNA isolation of fungi achieved using molecular biology kit (EZ-10 Spin Column Fungal Genomic DNA Mini-preps Kit, BIO BASIC INC., Canada) according to the instruction recommended by the manufacturer.
The ITS1-ITS4 primer pair was used to amplify the inverting 5.8S ribosomal DNA and the adjacent ITS1 and ITS2 regions. The ITS region on rDNA was amplified by using universal primers ITS1-(5’-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) (MWG-Biotech AG, Germany) which resulted in PCR product size of 471 bp for Fusarium oxysporum. Thermocycling program for isolated fungus was done in a GenAmp9700 thermal cycler (Perkin-Elmer) as following conditions: An initial denaturation set up at 94°C for 5 minutes was followed by 35 cycles of denaturation at 94°C for 1 minute annealing at 58°C for 1 minute and extension at 72°C for 1 minute with a final extension step of 72°C for 10 minutes. The PCR products were analysed by electrophoresing 10 μL of amplicons in 2% (w/v) agarose gel in TBE buffer {90 mM Tris, 90 mM boric acid, 2 mM EDTA, pH 8.3}, stained with ethidium bromide and supplied to the power at 75 volts for 90 minutes. The gel was visualised under computerised UV trans-illuminator and photographed. A 100 bp DNA marker was used as a reference to determine the size of fragments.
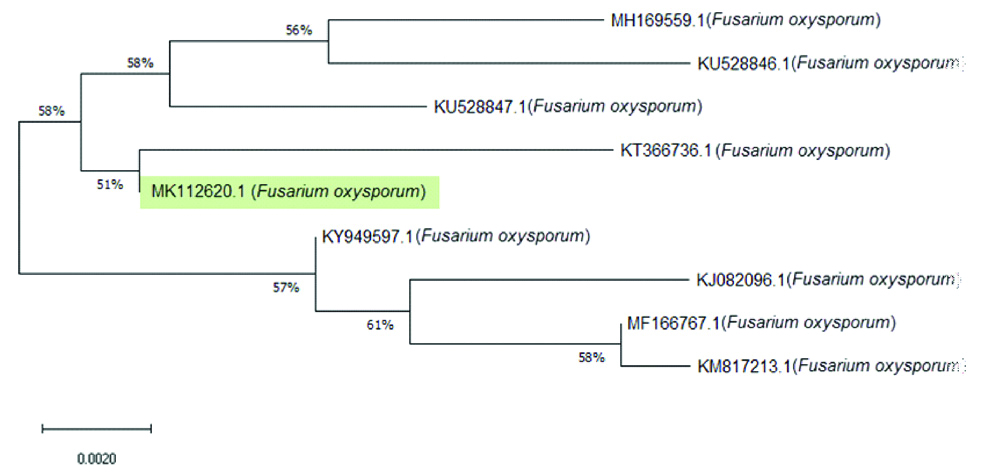
PCR product was sequenced by Macrogen Company (South korea). The ITS sequences of our isolate (MK112620) have been deposited in GenBank (NCBI). Used BLAST search tool, to compare the resulting sequences with sequences of rDNA accessed in Genbank, the phylogentic analysis for F. oxysporum showed that the obtained sequences shares 99% homology to Fusarium oxysporum strains: Indian isolates (KY949597, KM817213, MH169559 and MF166767), Tunisian isolates: (KU528846 and KU528847) and Chinese isolates: (KT366736 and KJ082096) [Table/Fig-4]. Together, morphological identification and molecular identification showed that Fusarium isolate was F. oxysporum (Genbank accession no. MK112620).
Neighbor-joining (NJ) tree of F. oxysporum based on nucleotide sequence of ITS-rDNA.
Discussion
Fusarium species are among the common hyaline non-dermatophytic causal agents related to onychomycosis [4]. Onychomycosis can be caused by several species of Fusarium such as F. oxysporum, F. solani, F. moniliforme and F. subglutinans [5]. The former two species were reported as the commonest among the genus as causal agents of onychomycosis [6].
According to studies by Balajee SA et al., on medically important Fusaria, revealed the presence of multiple cryptic species within each morphospecies [7], therefore, Fusarium oxysporum represents a complex. However, the ITS region is acceptable tool for identification of F. oxysporum [8].
Fusarium oxysporum complex is an a ubiquitous worldwide soil-borne facultative parasite inhabiting soil and plant debris [9], some isolates are well known plant pathogenic fungi mostly causing wilt diseases to a wide host range of vascular plants [10]. The fungus is also known as opportunistic pathogens to human and animals causing mostly superficial infections [11] as well as reported in disseminated infections mostly in immunocompromised patients [12]. Incidence of onychomycosis due to Fusarium oxysporum was commonly reported worldwide [6,13-15]. Several cases of onychomycosis due F. oxysporum were reported in immunocompetent and immunocompressed patients [16-18]. Present case study of onychomycosis was found in a 38-year-old female newly diagnosed with (of 10 months duration) acute myelogenous leukaemia. In Iraq, however, few studies have been published on onychomycosis and their aetiologic agents. Abdullah SK et al., diagnosed Epidermophyton floccosum, Trichophyton verrucosum, T. violaceum, Candida albicans, C. famata, C. parapsilosis and Geotrichum candidum as pathogens causing onychomycosis among patients from Basrah province, south Iraq [19]. In a subsequent study, Muhsin TM et al., reported C. albicans, C. parapsilosis and C. tropicalis as the frequent aetiologic agents of onychomycosis from the same city [20]. Hafidh RR and Abdulamir AS reported a case of white superficial onychomycosis related to Cladosporium sp. in patients from Baghdad [21]. Malassezia furfur was reported with 12% incidence as aetiologic agent in patients from Baghdad [22] and Fusarium sp. was reported with 33.3% in female patients from Tikrit city [23]. More recently, Auxarthron alboluteum was reported as aetiologic agent of toenail infection in a 63-year-old female patient from Kurdistan region of Iraq [3].
Conclusion(s)
In the present case, Fusarium oxysporum was reported as a new aetiologic agent of onychomycosis in Iraq. Identification of the species was confirmed by microscopic examination and sequencing of the ITS region of rDNA.
Author Declaration:
Financial or Other Competing Interests: No
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Sep 16, 2019
Manual Googling: Nov 30, 2019
iThenticate Software: Dec 28, 2019 (15%)
[1]. Kane J, Summerbell RC, Sigler L, Krajden SL, Laboratory handbook of dermatophytes 1997 Belmont CAStar Publishers [Google Scholar]
[2]. Leslie JF, Summerell BA, The Fusarium Laboratory Manual 2006 Ames, Iowa, USABlackwell Publishing10.1002/9780470278376 [Google Scholar] [CrossRef]
[3]. Gharib SJ, Abdullah SK, Richardson MD, Auxarthron alboleteum related to non-dermatophytic toenail infection in Kurdistan region, Iraq: A case reportMedical Mycology Case Reports 2019 26:53-56.10.1016/j.mmcr.2019.10.00631737472 [Google Scholar] [CrossRef] [PubMed]
[4]. Rankawaka RR, Nagahawatte A, Gunasekara TA, Fusarium onychomycosis: Prevalence, clinical presentations, response to itraconazole and terbinafine pulse therapy, and 1-year follow-up in nine casesInt J Dermatol 2015 54(11):1275-82.10.1111/ijd.1290626223159 [Google Scholar] [CrossRef] [PubMed]
[5]. Gaviria-Rivera A, Giraldo-Lopez A, Santa-Cardona C, Cano-Restrepo L, Molecular identification of clinical isolates of Fusarium in ColombiaRev Salud Publica 2018 20(1):94-102.10.15446/rsap.v20n1.5192330183891 [Google Scholar] [CrossRef] [PubMed]
[6]. Godoy P, Nunes F, Silva V, Tomimori-Yamashita J, Zaror L, Fischman O, Onychomycosis caused by Fusariumsolani and Fusariumoxysporum in Sao Paulo, BrazilMycopathologia 2004 157:287-90.10.1023/B:MYCO.0000024186.32367.d415180157 [Google Scholar] [CrossRef] [PubMed]
[7]. Balajee SA, Borman AM, Brandt ME, Cano J, Guenca-Esterella M, Dannaoui E, Sequence based identification of AspergillusFusarium and Mucorales species in the clinical mycology laboratory: Where are we and where should we go from hereJ Clin Microbiol 2009 47(4):877-84.10.1128/JCM.01685-0819073865 [Google Scholar] [CrossRef] [PubMed]
[8]. Irrnyi I, Serena C, Garcia-Hermosa D, Arabatziz M, Desonos-Ollivier M, Uv D, International Society of Human and Animal Mycology (ISHAM)-ITS reference DNA barcoding database- the quality controlled standard tool for routine identification of human and animal pathogenic fungiMedical Mycology 2015 53(4):313-37.10.1093/mmy/myv00825802363 [Google Scholar] [CrossRef] [PubMed]
[9]. Domsch KH, Gams W, Anderson TH, Compendium of soil fungi 1980 LondonAcademic Press [Google Scholar]
[10]. Armstrong GM, Armstrong JK, Formae speciales and races of Fusariumoxysporum causing wilt disease. In, Nelson PE, Toussoun TA and Cook RJ (Eds)Fusarium: Disease, Biology, and Taxonomy 1981 Pennsylvania State University Press:391-99. [Google Scholar]
[11]. Gupta AK, Baran R, Summerbell RC, Fusarium infections of the skinCurr Opin Infect Dis 2000 13(2):121-28.10.1097/00001432-200004000-00005 [Google Scholar] [CrossRef]
[12]. Nucci M, Anaissie E, Fusarium infections in immunocompromised patientsClin Microbiol Rev 2007 20(4):695-704.10.1128/CMR.00014-0717934079 [Google Scholar] [CrossRef] [PubMed]
[13]. Rush-Munro FM, Black H, Dingley JM, Onychomycosis caused by FusariumoxysporumAustralasian Journal of Dermatology 1971 12(1):18-29.10.1111/j.1440-0960.1971.tb00688.x4254822 [Google Scholar] [CrossRef] [PubMed]
[14]. DiSalvo AF, Fickling AM, A case of nondermatophytic toe onychomycosis caused by Fusarium oxysporumArch Dermatol 1980 116(6):699-700.10.1001/archderm.1980.016403000870276445716 [Google Scholar] [CrossRef] [PubMed]
[15]. Romano C, Miracco C, Difonza EM, Skin and nail infections due to Fusariumoxysporum in Tuscant, ItalyMycoses 1998 41(9-10):433-37.10.1111/j.1439-0507.1998.tb00369.x9916472 [Google Scholar] [CrossRef] [PubMed]
[16]. Guilhermetti E, Takahachi G, Shinobu CS, Svidzinski TIE, Fusarium species as agents of onychomycosis in immunocompetent hostsInt J Dermatol 2007 46(8):822-26.10.1111/j.1365-4632.2007.03120.x17651164 [Google Scholar] [CrossRef] [PubMed]
[17]. Shah SR, Dalal BD, Modak MS, Nondermatophytic onychomycosis by Fusariumoxysporum in an immunocompetent hostJournal de Mycologie Medicale 2016 26:e18-e21.10.1016/j.mycmed.2015.12.003 [Google Scholar] [CrossRef]
[18]. Carvalho VO, Vicente VA, Werner B, Gomes RR, Fornari G, Herkert PF, Onychomycosis by Fusarium oxysporum probably acquired in uteroMedical Mycology Case Reports 2014 6:58-61.10.1016/j.mmcr.2014.09.00525383318 [Google Scholar] [CrossRef] [PubMed]
[19]. Abdullah SK, Al-Hamdani FM, Naama MS, Incidence and aetiologic study of onychomycosis in Basrah, IraqIraqi Journal of Biology 2002 2(2):464-68. [Google Scholar]
[20]. Muhsin TM, Al-Rubaiy Al-Duboon Characteristics of dermatophytoses in Basrah, IraqMycoses 2002 :335-38.Available at: https://doi.org/10.1046/j.1439-0507.1999.00463.x10.1046/j.1439-0507.1999.00463.x10424106 [Google Scholar] [CrossRef] [PubMed]
[21]. Hafidh RR, Abdulamir AS, Cladosporium spp. as a causative agent of white superficial onychomycosisEast Mediterr Health J 2008 14(1):231-33. [Google Scholar]
[22]. Ammari AM, Talib NT, Hussein AF, Abdul-Rahman ES, Association of Malasseziafurfur with onychomycosis patients in Baghdad, IraqDiyala Journal of Medicine 2018 14(1):115-22.10.26505/DJM.14013741011 [Google Scholar] [CrossRef]
[23]. AL-Tikrity Thekra A, AL-Juboor Osama M, Onychomycosis in females; a clinico-mycological studyTikrit Medical Journal 2011 17(1):43-44. [Google Scholar]