Introduction
Hyperprolactinemia is one of the most common hypothalamic-pituitary dysfunctions where serum prolactin levels increase beyond normal range. Studies have suggested association of heavy metals with prolactin levels.
Aim
To investigate the association of serum levels of heavy metals with prolactin levels in hyperprolactinemia patients.
Materials and Methods
A total of 102 hyperprolactinemia patients (>100 ng/mL serum prolactin levels) and 25 controls were included in the study. Hyperprolactinemia patients were classified into macroprolactinemia (n=22) and true hyperprolactinemia (n=80) based on post Poly Ethylene Glycol (PEG) recovery of prolactin of <25%. Serum Cadmium (Cd), Chromium (Cr), Manganese (Mn) and Lead (Pb) levels were analysed using Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) method. Statistical analysis was done using SPSS version 21.0 and Stata version 14.2. Student’s t-test and Pearson correlation were used. The p<0.05 was considered statistically significant.
Results
There was no significant correlation between serum levels of prolactin and heavy metals Cd (r=0.067, p=0.457), Cr (r=-0.065, p=0.465), Mn (r=-0.076, p=0.393) and Pb (r=-0.148, p=0.097). No significant difference was found in serum levels of heavy metals between macroprolactinemia and true hyperprolactinemia patients (p=0.521, 0.690, 0.564 and 0.488 for Cd, Cr, Mn and Pb, respectively). ROC analysis also did not reveal any significance in any of the four heavy metals studied.
Conclusion
The results suggest that probably there is no association of serum prolactin levels, macroprolactinemia or hyperprolactinemia with heavy metals.
Introduction
Heavy metals in the environment enter our body through various sources, get accumulated in brain and interfere in the normal functioning of hypothalamic-pituitary-gonadal axis [1]. Studies have shown role of dopaminergic system in heavy metal neurotoxicity [2,3]. Heavy metals also act as xenoestrogens, mimicking the action of oestrogen in body and activating cell responses normally mediated by oestrogen [4].
Prolactin is produced by lactotroph cells of anterior pituitary and performs more than 300 roles including reproduction, metabolism, immune response, homeostasis and angiogenesis [5]. Prolactin secretion is regulated by hypothalamic-pituitary-gonadal axis. Dopaminergic system is inhibitory to prolactin secretion [6]. Estradiol stimulates lactotroph cell proliferation in the anterior pituitary and increases secretion of prolactin [7]. Hyperprolactinemia is one of the most common hypothalamic-pituitary dysfunctions where serum prolactin levels increase beyond normal range [8]. Hyperprolactinemia is a major cause of galactorrhoea, irregular menses and infertility in young women and loss of libido and infertility in men [9]. The condition in hyperprolactinemia where large immune-complex macroprolactin molecules exist as major molecular form of prolactin in sera is called macroprolactinemia [10]. Besides the known causes of pituitary adenoma, drug induced and secondary causes like chronic kidney disease, 16-35% hyperprolactinemia cases remain idiopathic [11,12]. Several studies have investigated prolactin levels in environmental and occupational exposure to heavy metals and have found significant relations [13-17].
Cadmium (Cd) is a heavy metal with role in signal transduction and inhibition of gene expression [18]. Cadmium, at nanomolar concentrations, displayed xenoestrogenic activities by affecting lactotroph proliferation and hormone release from anterior pituitary cells [4], while at micromolar concentrations, is cytotoxic and inhibits prolactin release [13]. Chromium (Cr) has a toxic effect on hypothalamus-pituitary-gonadal axis. Chromium (VI) accumulates in pituitary and causes a reduction in serum prolactin levels in vivo. Cr (VI) causes apoptosis of lactotroph cells in vitro by generation of reactive oxygen species [14]. Exposure to Manganese (Mn) was shown to increase prolactin levels [15]. However, study on prolactinoma patients found significantly lower Mn content in RBCs compared to controls, suggesting a relation between hyperprolactinemia and Mn levels via oestrogen regulation [16]. Lead (Pb) is one of the oldest environmental poisons having toxic effects due to its ability to substitute calcium and accumulation in brain and bone for long time [17]. The possible inhibitory effect of lead on dopaminergic pathway was observed in Pb exposed male workers having high prolactin levels [2].
The association of heavy metals with prolactin levels point towards a potential relation of heavy metals with hyperprolactinemia condition also. Therefore, the present study was aimed to find correlation of prolactin levels with heavy metals Cd, Cr, Mn and Pb in hyperprolactinemia patients and to compare levels of heavy metals between macroprolactinemia and true hyperprolactinemia as the latter are more commonly associated with the symptoms of hyperprolactinemia.
Materials and Methods
This was a cross-sectional pilot study conducted between June 2014-February 2017. The study was approved by Institutional ethics committee (Ref. No.: IESC/T-63/21.01.2015, RT-39/01.04.2015). Written informed consent was taken from each participant before inclusion in the study. The study included 102 hyperprolactinemia patients with prolactin level >100 ng/mL in two occasions of >1 month interval aged 19-48 years recruited from Reproductive Biology department of a tertiary care hospital. Women with physiological hyperprolactinemia were excluded from the study. A total of 25 age-matched healthy women recruited from the general community during the same period of time having normal prolactin level were included as controls. As a causation and to check the accuracy of the kit, 25 healthy women were taken as controls. Detailed clinical and medical history was taken as per pre-determined proforma. The minimum initial evaluation included complete medical history, physical examination, hormone measurements and CT/MRI for prolactinoma or other pituitary adenoma. Prolactin assays were done on highly specific Chemiluminescence Microparticle Immunoassay (CMIA) (7K76 G6-5314/R06 B7K760) using ARCHITECT PLUS i2000SR automated immunoassay system (Abbott Laboratories, USA).
About 2 mL peripheral blood was collected from each participant in plain vial and was allowed to coagulate at room temperature for 30 minutes. Serum samples were obtained by centrifuging blood sample at 5000 rpm for 5 minutes at room temperature and were stored at -80°C until further analysis. PEG precipitation was done with PEG 6000 (Catalogue# SC- 302016) to classify hyperprolactinemia patients into macroprolactinemia and true hyperprolactinemia based upon post PEG precipitation recovery of prolactin of <25% [12].
Serum levels of Cd, Cr, Mn and Pb were measured by ICP-AES (Model JY 2000, HORIBA JobinYvon, France) at Department of Pharmacology, AIIMS, New Delhi, India. The limits of detection for serum heavy metals were as follows: Cd, 0.35 μg/L; Cr, 0.5 μg/L; Mn, 0.3 μg/L; Pb, 5 μg/L [19]. Wavelengths for each heavy metal, selected from a pre-defined set using the ICP software version 5.2, were: Cd, 228.56 nm; Cr, 283.56 nm; Mn, 257.61 nm and Pb, 220.35 nm. One mL serum was mixed with 4 mL conc. HNO3 and 1 mL H2O2 in digestion cylinders. The samples were digested as per the cycle: temperature 70°C, rinse time 5 minutes, hold time 2 minutes; temperature 100°C, rinse time 5 minutes, hold time 5 minutes; temperature 130°C, rinse time 5 minutes, hold time 5 minutes. The digested samples were allowed to cool at room temperature followed by dilution to 10 mL volume with double distilled milliQ. The diluted samples were run in triplicate for heavy metal estimation by ICP-AES along with the multi-element standard of concentration 100 parts per billion (ppb) which was run after every 15 samples.
Statistical Analysis
Statistical analysis was performed using IBM Statistical Package for the Social Sciences (SPSS) software, version 21.0. Results were expressed as mean±SD (range) and median (IQR). Student’s t-test was performed to compare mean between macroprolactinemia and true hyperprolactinemia patients. Pearson correlation coefficient was used to find correlation between heavy metals and prolactin. The p<0.05 was considered statistically significant. ROC curve was performed on Stata version 14.2.
Results
Total 102 hyperprolactinemia patients were included of which 98 (96.08%) were females and 4 (3.92%) were males. Mean age of hyperprolactinemia patients was 30.61±6.6 (range 19-48) years. Prolactin levels varied greatly among hyperprolactinemia patients, ranging from 100 to 8484 ng/mL, with median prolactin level 159.17 ng/mL (128.43, 245.36). The demographic, aetiologic and clinical profile of hyperprolactinemia patients are presented in [Table/Fig-1,2 and 3], respectively.
Demographic profile of hyperprolactinemia patients.
| Hyperprolactinemia patients (N=102) |
|---|
| Age (years), mean±SD | 30.6±6.6 |
| Males, n (%) | 4 (3.9) |
| Females, n (%) | 98 (96.1) |
| Married, n (%) | 86 (84.3) |
| No. of smokers, n (%) | 2 (2.0) |
| No. of alcoholics, n (%) | 4 (3.9) |
Aetiological details of hyperprolactinemia patients.
| Hyperprolactinemia patients (N=102) |
|---|
| Pituitary adenoma, n (%) | 19 (18.6) |
| Drug induced, n (%) | 36 (35.3) |
| Idiopathic, n (%) | 36 (35.3) |
| Other secondary causes, n (%) | 11 (10.8) |
| Macroprolactinemia, n (%) | 22 (21.6) |
| True hyperprolactinemia, n (%) | 80 (78.4) |
Clinical profile of hyperprolactinemia patients.
| Hyperprolactinemia patients (N=102) |
|---|
| Respiratory disease/Asthma, n (%) | 0 (0) |
| Hypertension, n (%) | 8 (7.8) |
| Diabetes, n (%) | 1 (1.0) |
| Renal disease, n (%) | 10 (9.8) |
| Adrenal disorder, n (%) | 0 (0) |
| Thyroid disorder*, n (%) | 20 (19.6) |
| Bone/Joint pain, n (%) | 55 (53.9) |
| Body mass index, mean±SD | 23.9±4.7 |
| Prolactin (ng/mL), median (IQR) | 159.17 (128.43, 245.36) |
| TSH (mU/L), median (IQR) | 2.58 (1.6, 3.8) |
*14 patients euthyroid with thyroid medication; 4 patients hypothyroid without thyroid medication; 1 patient hypothyroid with thyroid medication; 1 patient euthyroid with goiter
The mean age of 25 healthy control females was 27±4.7 (range 20-39) years and mean prolactin levels 11.8±4.2 ng/mL. The serum levels of Cd, Cr, Mn and Pb in controls were 14.3±0.8 ppb; 189.3±20.2 ppb; 33.3±4.3 ppb and 20.8±8.1 ppb, respectively.
The present study found no significant correlation between serum levels of Cd, Cr, Mn and Pb with prolactin levels in hyperprolactinemia patients and controls [Table/Fig-4].
Pearson correlation of serum levels of heavy metals with prolactin levels.
| Variable | Prolactin |
|---|
| Cadmium |
| Pearson correlation | 0.067 |
| Sig. (2-tailed) | 0.457 |
| N | 127 |
| Chromium |
| Pearson correlation | -0.065 |
| Sig. (2-tailed) | 0.465 |
| N | 127 |
| Manganese |
| Pearson correlation | -0.076 |
| Sig. (2-tailed) | 0.393 |
| N | 127 |
| Lead |
| Pearson correlation | -0.148 |
| Sig. (2-tailed) | 0.097 |
| N | 127 |
Out of the 102 hyperprolactinemia patients 22 were macroprolactinemia and 80 were true hyperprolactinemia patients based upon the presence of macroprolactin fraction of >75% in total prolactin as estimated by post PEG precipitation recovery of prolactin of <25%. Comparison of serum levels of heavy metals between macroprolactinemia and true hyperprolactinemia found no significant difference in any heavy metal studied between the two groups [Table/Fig-5].
Comparison of serum levels of heavy metals Cd, Cr, Mn and Pb between macroprolactinemia and true hyperprolactinemia.
| Variable | Macroprolactinemia (N=22) | True Hyperprolactinemia (N=80) | p-value |
|---|
| Prolactin (ng/mL) |
| Median (IQR) | 137.6 (115.43, 189.88) | 164.02 (130.91, 257.58) | 0.0543 |
| (Range) | (100-566.3) | (100-8484) |
| Cadmium (ppb) |
| Mean±SD | 14.16±1.01 | 14.30±0.84 | 0.521 |
| (Range) | (12.7-17.7) | (12.9-18.1) |
| Chromium (ppb) |
| Mean±SD | 171.42±12.41 | 169.44±22.35 | 0.690 |
| (Range) | (149.9-208.0) | (9.6-220.4) |
| Manganese (ppb) |
| Mean±SD | 31.28±1.81 | 30.95±2.56 | 0.564 |
| (Range) | (27.9-36.9) | (13.2-36.3) |
| Lead (ppb) |
| Mean±SD | 19.76±5.30 | 18.85±5.47 | 0.488 |
| (Range) | (12.2-31.3) | (6.7-30.6) |
p<0.05 considered significant
Cd had AUC 0.4388 with CI: 0.305-0.572. Cr had AUC 0.218 with CI: 0.122-0.314. Mn had AUC 0.307 with CI: 0.177-0.437. Pb had AUC 0.393 with CI: 0.252-0.534. None of the four heavy metals showed any significance in ROC analysis [Table/Fig-6].
ROC curve analysis of Cd, Cr, Mn and Pb between hyperprolactinemia patients and controls.
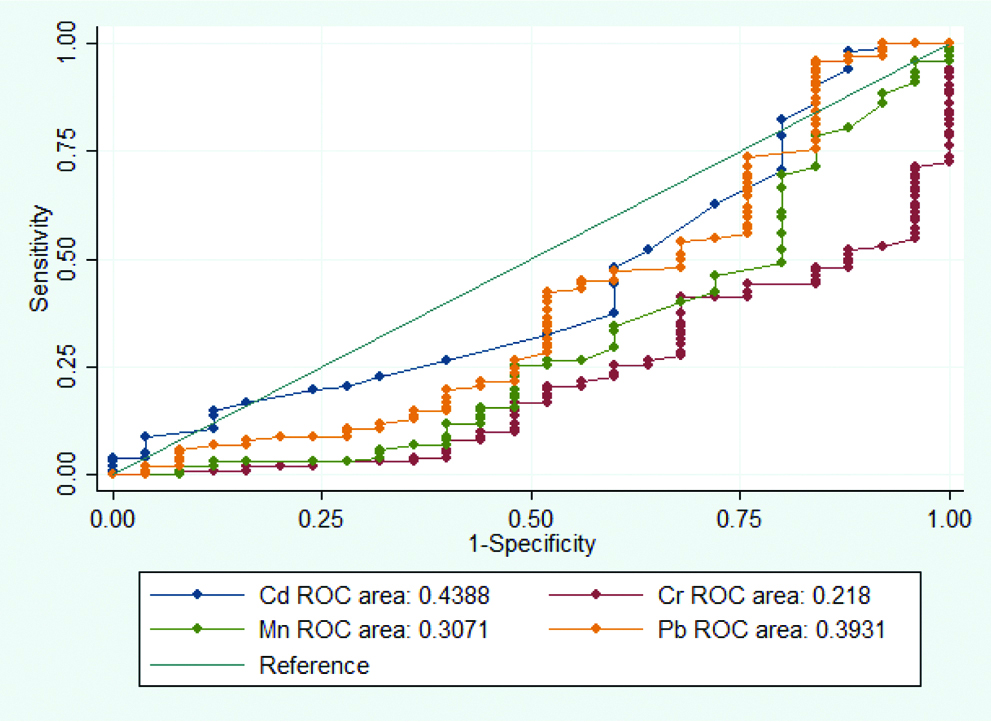
Discussion
Many studies have compared levels of heavy metals with prolactin levels in different target groups and have suggested effect of heavy metals on prolactin levels but none of the studies have compared levels of heavy metals in pathological hyperprolactinemia patients to probe whether heavy metals actually have role in pathologically raised prolactin levels [13-17]. The present study is unique as it analysed heavy metal levels in hyperprolactinemia patients to check association of heavy metals with hyperprolactinemia.
The present study found no significant correlation between serum levels of Cd, Cr, Mn and Pb with prolactin levels in hyperprolactinemia patients and controls. No statistically significant difference was found in any of the four heavy metals studied between macroprolactinemia and true hyperprolactinemia patients either. ROC analysis, also, did not reveal any significance in Cd, Cr, Mn or Pb in hyperprolactinemia patients and controls.
The present study findings are supported by the study of Leite EMA et al., who did not find any significant correlation between blood Pb and serum prolactin levels in children with low environmental exposure to Pb, although the target group in the study varied from the present study [20].
The present findings are contradictory to many other studies presenting correlation between prolactin and heavy metals. Study on non-occupational group of men showed inverse correlation of Cd, Mn and Pb while positive correlation of chromium with prolactin [21]. Chromium levels have been inversely related to prolactin levels [14]. Study on rats showed that hexavalent chromium caused adverse effects on anterior pituitary and resulted in reduced serum prolactin levels [14]. In-vitro studies have shown that Cr (VI) decreases prolactin levels by producing oxidative stress which causes apoptosis of prolactin secreting lactotroph cells [22,23]. Occupational exposure to Mn has been shown to be positively correlated to prolactin levels [24]. The whole blood Mn concentration was associated with serum prolactin concentrations as shown by higher prolactin in welders compared to referents [24]. Study in rats presented that Mn exposure causes increase in prolactin by decreasing dopamine and Pit-1 which are regulators of both prolactin and dopamine [25]. Study on children had similar inverse relation between Pb and prolactin [26]. On the other hand, there are studies which showed significantly higher plasma prolactin values in Pb exposed workers [27]. Lucchini R et al., found high prolactin levels in male workers with occupational exposure to Pb [2].
The contrasting results in present study compared to others may be due to many factors. None of the earlier studies had hyperprolactinemia patients as the target group like the present study. Also, the same metal can have different effects on prolactin levels based upon the exposure dose [28], and unlike present study, most of the studies are done on occupational exposure of heavy metals where the exposure dose was very high compared to non-occupational environmental exposure.
Further, the inconsistency in association of prolactin levels with heavy metals in different studies may suggest potentially differing sites and mechanisms of actions of heavy metals [21]. Heavy metals can affect prolactin at different levels: directly at the cellular level [29], at dopaminergic level-by affecting dopamine receptor sensitivity and number in tubero-infundibular region, or by inhibiting release of dopamine in synaptosomal region [30,31]. In addition to these, prolactin secretion is also regulated by other neurotransmitters including Gamma-Aminobutyric Acid (GABA), glycine and glutamate [29], which might also be targeted by heavy metals affecting prolactin levels [32]. Effect of heavy metals on prolactin levels can also occur via xenoestrogenic property of heavy metals as oestrogen is known to up-regulate expression of prolactin gene; synthesis, storage and release of prolactin as well as proliferation of lactotroph cells in pituitary [7].
True hyperprolactinemia patients are usually associated with clinical symptoms of hyperprolactinemia. So, the study also compared heavy metals between true hyperprolactinemia and macroprolactinemia patients, which are big immune-complexes of prolactin and are not always associated with clinical symptoms of hyperprolactinemia. No significant difference was observed in heavy metals between macroprolactinemia and true hyperprolactinemia patients suggesting no differential role of heavy metals in true hyperprolactinemia or macroprolactinemia. We did not find any other study comparing heavy metals between macroprolactinemia and true hyperprolactinemia groups.
This suggests that although studies have reported positive (or inverse) association of heavy metals with prolactin levels in occupational and non-occupational groups, but hyperprolactinemia condition may not be associated with heavy metal levels.
Limitation(s)
One potential limitation of the study was that the study population was heterogenous with respect to the cause of hyperprolactinemia. This might have generated confounding factors contributing to the present findings.
Conclusion(s)
No significant correlation was observed between serum levels of prolactin and heavy metals in hyperprolactinemia patients. Also, levels of heavy metals did not vary significantly between macroprolactinemia and true hyperprolactinemia patients. Thus, probably there was no association of prolactin levels, macroprolactinemia or hyperprolactinemia with heavy metal levels of Cd, Cr, Mn and Pb. However, these findings should be validated in a large cohort of hyperprolactinemia patients.
*14 patients euthyroid with thyroid medication; 4 patients hypothyroid without thyroid medication; 1 patient hypothyroid with thyroid medication; 1 patient euthyroid with goiter
p<0.05 considered significant
[1]. Popek W, Dietrich G, Glogowski J, Demska-Zakeś K, Drag-Kozak E, Sionkowski J, Influence of heavy metals and 4-nonylphenol on reproductive function in fishReprod Biol 2006 6(Suppl 1):175-88. [Google Scholar]
[2]. Lucchini R, Albini E, Cortesi I, Placidi D, Bergamaschi E, Traversa F, Assessment of neurobehavioral performance as a function of current and cumulative occupational lead exposureNeurotoxicology 2000 21(5):805-11. [Google Scholar]
[3]. Jones DC, Miller GW, The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addictionBiochem Pharmacol 2008 76(5):569-81.10.1016/j.bcp.2008.05.01018555207 [Google Scholar] [CrossRef] [PubMed]
[4]. Ronchetti SA, Miler EA, Duvilanski BH, Cabilla JP, Cadmium mimics estrogen-driven cell proliferation and prolactin secretion from anterior pituitary cellsPloS One 2013 8(11):e8110110.1371/journal.pone.008110124236210 [Google Scholar] [CrossRef] [PubMed]
[5]. Freeman ME, Kanyicska B, Lerant A, Nagy G, Prolactin: Structure, function, and regulation of secretionPhysiol Rev 2000 80(4):1523-631.10.1152/physrev.2000.80.4.152311015620 [Google Scholar] [CrossRef] [PubMed]
[6]. Gudelsky GA, Nansel DD, Porter JC, Role of estrogen in the dopaminergic control of prolactin secretionEndocrinology 1981 108(2):440-44.10.1210/endo-108-2-4407449733 [Google Scholar] [CrossRef] [PubMed]
[7]. Spady TJ, McComb RD, Shull JD, Estrogen action in the regulation of cell proliferation, cell survival, and tumorigenesis in the rat anterior pituitary glandEndocrine 1999 11(3):217-33.10.1385/ENDO:11:3:217 [Google Scholar] [CrossRef]
[8]. Torre DL, Falorni A, Pharmacological causes of hyperprolactinemiaTher Clin Risk Manag 2007 3(5):929-51. [Google Scholar]
[9]. Glezer A, Bronstein MD, Hyperprolactinemia. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, et al., editorsEndotext [Internet] 2000 [cited 2018 Oct 22] South Dartmouth (MA)MDText.com, IncAvailable from: http://www.ncbi.nlm.nih.gov/books/NBK278984/ [Google Scholar]
[10]. Shimatsu A, Hattori N, Macroprolactinemia: diagnostic, clinical, and pathogenic significanceClin Dev Immunol 2012 2012:16713210.1155/2012/16713223304187 [Google Scholar] [CrossRef] [PubMed]
[11]. Adra A, El Zibdeh MY, Abdul Malek AMM, Hamrahian AH, Abdelhamid AMS, Colao A, Differential diagnosis and management of abnormal uterine bleeding due to hyperprolactinemiaMiddle East Fertil Soc J [Internet] 2016 Sep 1 [cited 2018 Oct 12] 21(3):137-47.Available from: http://www.sciencedirect.com/science/article/pii/S111056901530093510.1016/j.mefs.2016.02.001 [Google Scholar] [CrossRef]
[12]. Kalsi AK, Halder A, Jain M, Chaturvedi PK, Sharma JB, Prevalence and reproductive manifestations of macroprolactinemiaEndocrine [Internet] 2019 63(2):332-40.Available from: https://doi.org/10.1007/s12020-018-1770-610.1007/s12020-018-1770-630269265 [Google Scholar] [CrossRef] [PubMed]
[13]. Poliandri AH, Cabilla JP, Velardez MO, Bodo CC, Duvilanski BH, Cadmium induces apoptosis in anterior pituitary cells that can be reversed by treatment with antioxidantsToxicol Appl Pharmacol 2003 190(1):17-24.10.1016/S0041-008X(03)00191-1 [Google Scholar] [CrossRef]
[14]. Quinteros FA, Poliandri AHB, Machiavelli LI, Cabilla JP, Duvilanski BH, In vivo and in vitro effects of chromium VI on anterior pituitary hormone release and cell viabilityToxicol Appl Pharmacol 2007 218(1):79-87.10.1016/j.taap.2006.10.01717141818 [Google Scholar] [CrossRef] [PubMed]
[15]. Ellingsen DG, Haug E, Gaarder PI, Bast-Pettersen R, Thomassen Y, Endocrine and immunologic markers in manganese alloy production workersScand J Work Environ Health 2003 29(3):230-38.10.5271/sjweh.72612828393 [Google Scholar] [CrossRef] [PubMed]
[16]. Nishi Y, Aihara K, Hatano S, Kihara M, Ohta M, Sakoda K, Concentrations of zinc, copper, manganese and selenium in blood and urine of patients with prolactinomaActa Paediatr Jpn 1988 30(2):199-203.10.1111/j.1442-200X.1988.tb02519.x3149853 [Google Scholar] [CrossRef] [PubMed]
[17]. Takser L, Mergler D, Lafond J, Very low level environmental exposure to lead and prolactin levels during pregnancyNeurotoxicol Teratol 2005 27(3):505-08.10.1016/j.ntt.2005.03.00915939210 [Google Scholar] [CrossRef] [PubMed]
[18]. Waisberg M, Joseph P, Hale B, Beyersmann D, Molecular and cellular mechanisms of cadmium carcinogenesisToxicology 2003 192(2-3):95-117.10.1016/S0300-483X(03)00305-6 [Google Scholar] [CrossRef]
[19]. Jain M, Kalsi AK, Srivastava A, Gupta YK, Halder A, High Serum Estradiol and Heavy Metals Responsible for Human Spermiation Defect-A Pilot StudyJ Clin Diagn Res JCDR 2016 10(12):RC09-RC13.10.7860/JCDR/2016/22483.899028208955 [Google Scholar] [CrossRef] [PubMed]
[20]. Leite EMA, Leroyer A, Nisse C, Haguenoer JM, de Burbure CY, Buchet JP, Urinary homovanillic acid and serum prolactin levels in children with low environmental exposure to leadBiomark Biochem Indic Expo Response Susceptibility Chem 2002 7(1):49-57.10.1080/13547500110074419212101784 [Google Scholar] [CrossRef] [PubMed]
[21]. Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, Multiple metals predict prolactin and thyrotropin (TSH) levels in menEnviron Res 2009 109(7):869-73.10.1016/j.envres.2009.06.00419595304 [Google Scholar] [CrossRef] [PubMed]
[22]. Quinteros FA, Machiavelli LI, Miler EA, Cabilla JP, Duvilanski BH, Mechanisms of chromium (VI)-induced apoptosis in anterior pituitary cellsToxicology 2008 249(2-3):109-15.10.1016/j.tox.2008.04.01218547707 [Google Scholar] [CrossRef] [PubMed]
[23]. Nudler SI, Quinteros FA, Miler EA, Cabilla JP, Ronchetti SA, Duvilanski BH, Chromium VI administration induces oxidative stress in hypothalamus and anterior pituitary gland from male ratsToxicol Lett 2009 185(3):187-92.10.1016/j.toxlet.2009.01.00319167472 [Google Scholar] [CrossRef] [PubMed]
[24]. Ellingsen DG, Chashchin V, Haug E, Chashchin M, Tkachenko V, Lubnina N, An epidemiological study of reproductive function biomarkers in male weldersBiomark Biochem Indic Expo Response Susceptibility Chem 2007 12(5):497-509.10.1080/1354750070136649617701748 [Google Scholar] [CrossRef] [PubMed]
[25]. Kim HY, Lee CK, Lee JT, Moon CS, Ha SC, Kang SG, Effects of manganese exposure on dopamine and prolactin production in ratNeuroreport 2009 20(1):69-73.10.1097/WNR.0b013e328315cd3519057282 [Google Scholar] [CrossRef] [PubMed]
[26]. de Burbure C, Buchet JP, Leroyer A, Nisse C, Haguenoer JM, Mutti A, Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levelsEnviron Health Perspect 2006 114(4):584-90.10.1289/ehp.820216581550 [Google Scholar] [CrossRef] [PubMed]
[27]. Govoni S, Battaini F, Fernicola C, Castelletti L, Trabucchi M, Plasma prolactin concentrations in lead exposed workersJ Environ Pathol Toxicol Oncol 1987 7(4):13-15. [Google Scholar]
[28]. Lafuente A, Cano P, Esquifino A, Are cadmium effects on plasma gonadotropins, prolactin, ACTH, GH and TSH levels, dose-dependent?Biometals 2003 16(2):243-50.10.1023/A:102065812841312572682 [Google Scholar] [CrossRef] [PubMed]
[29]. Alessio L, Lucchini R, Prolactin changes as a consequence of chemical exposureEnviron Health Perspect 2006 114(10):A573-74.author reply A57410.1289/ehp.114-a57317035115 [Google Scholar] [CrossRef] [PubMed]
[30]. Silbergeld EK, Chisolm JJ Jr, Lead poisoning: Altered urinary catecholamine metabolites as indicators of intoxication in mice and childrenScience 1976 192(4235):153-55.10.1126/science.12577631257763 [Google Scholar] [CrossRef] [PubMed]
[31]. Pokora MJ, Richfield EK, Cory-Slechta DA, Preferential vulnerability of nucleus accumbens dopamine binding sites to low-level lead exposure: time course of effects and interactions with chronic dopamine agonist treatmentsJ Neurochem 1996 67(4):1540-50.10.1046/j.1471-4159.1996.67041540.x8858938 [Google Scholar] [CrossRef] [PubMed]
[32]. Catalano PN, Bonaventura MM, Silveyra P, Bettler B, Libertun C, Lux-Lantos VA, GABA(B1) knockout mice reveal alterations in prolactin levels, gonadotropic axis, and reproductive functionNeuroendocrinology 2005 82(5-6):294-305.10.1159/00009312816682806 [Google Scholar] [CrossRef] [PubMed]