Carcinogenesis is a multistep process that comprises cellular proliferation, differentiation, apoptosis and a complex interplay between intricate processes that determine tumour growth and progression [1-3]. During this process, to function efficiently, the tumour cells show an expanded repertoire to accumulate biomass components that support their growth and improve survival [2,4-7]. This adaptation of tumour cells to the harsh tumour microenvironment is called “metabolic reprogramming” which has been considered as a hallmark of cancer [2,6,7].
Lipids constitute the basic structure of cell membrane that function as energy sources and signalling molecules during normal cell proliferation. An increased rate of lipid synthesis including TC, lipoproteins and TG has been recognised as an important aspect of transformed cells in malignancy [2-5]. The highly proliferative cancerous over activate their endogenous synthesis and increase the consumption of exogenous (or dietary) lipids to satiate their avidity towards lipid and cholesterol [3,6,7].
Till date, Oral cancer remains the most common cancer in Central and South East Asian countries [4,8]. The Global Adult Tobacco Survey (GATS) 2010, has recorded that nearly 34.6% of Indian population consume tobacco of which 29.1% consume tobacco daily [4]. Oral cancer is almost always preceded by Oral Potentially Malignant Disorders (OPMDs) including leukoplakia, erythroplakia and oral submucous fibrosis, its early detection before malignant transformation still remains a matter of concern [1,3,4,9].
Clinical oral examination remains the mainstay for the early detection of OPMD’s though; various biochemical and molecular markers have been proposed as adjuvants for identifying oral cancer or malignant transformation of OPMD’s, but they lack validation and a number of drawbacks still remain unaddressed [8,10].
An imperative issue in the research in the field of oral medicine and pathology is developing novel diagnostic tools and markers. Saliva is one such new age diagnostic tool. It is easy to collect with minimal risk for health professionals and better patient acceptance, convenient to store and dispatch without the use of any sophisticated equipment [11-15].
Accumulating evidence has been described that lipid and cholesterol associated pathways are substantially reprogrammed in cancer cells [1,3-5,16-19]. Quantitative alteration in serum lipid profile has been considered as one of the biochemical marker in tobacco chewers with OPMD’s and malignancies [1,4,5,9,16,17,19]. The purpose of such alterations is unclear as to whether hypocholesterolemia is the cause of cancer or its result. Also, there is insufficient literature to assess the exact mechanism of dyslipidemia [1,19].
There are not many studies that have been conducted for analysing the role of salivary lipid profile alterations in oral cancer and OPMD’s and its reliability in early diagnosis of cancer. This salivary lipid profile could serve as a simple and cost-effective alternative to the serum lipid profile for early diagnosis of malignant transformation of OPMD’s. So, the present study was undertaken as a preliminary assessment aimed to evaluate and correlate the salivary lipid profiles in tobacco chewers.
Materials and Methods
This cross sectional study was conducted in the Department of Oral Medicine and Radiology, Sinhgad Dental College and Hospital, Pune from May to July 2019. The study was approved by the Institutional Ethical and Research Committee (SDCH/SAC/2017-18/140). The sample consisted of total of 30 subjects (15 tobacco chewers and 15 age and sex matched controls) aged 20-40 years were included in the study. The written informed consent was obtained from all the subjects. All clinical examinations were carried out by a single trained examiner. Detailed case history including the diet history, habits history, extraoral and intraoral examination was performed as per the case history proforma. Individuals who had no complaint or any major illness in recent past were included in the study. Individuals showing clinical signs of periodontitis, medically compromised patients, patients with other systemic illness like diabetes, hypertension, uremia, nephrotic syndrome, thyroid disorders, liver dysfunction and also patients on medication including steroids that can alter the lipid levels were excluded from the study.
Method of Collection of Saliva
Unstimulated saliva samples (2 mL) were collected from each patient after overnight fasting (12 hr) under resting conditions by asking the patients to lean forward and spit the saliva in epindroff tubes following rinsing of mouth with distilled water. The patients were given detailed information about the collection protocol: they were asked to follow proper brushing technique (BASS) without using toothpaste. They were explained the importance of the exact timing of the collection of samples and were also instructed to avoid fluid ingestion and chewing gum for at least 30 minutes before collection.
Lipid Analysis
Lipid analysis was done on a fully automated analyser based on spectrophotometric principle using kits obtained from Mindray BS 200. The salivary lipid profile was analysed on the same day of the collection of saliva [12].
The salivary TC and salivary TG levels were estimated by taking 10 μL of distilled water, 10 μL of sample and 10 μL of cholesterol standard in separate test tubes.
The salivary High Density Lipoprotein (HDL) level was estimated by mixing 250 μL of saliva sample with 500 μL of HDL-precipitating reagent in separate test tubes, followed by 10 minutes incubation at room temperature. Mixtures were centrifuged at 4000 rpm for 10 minutes to obtain a clear supernatant. In separate test tubes, 50 μL of distilled water, 50 μL of supernatant and 50 μL of HDLC were taken.
To all test tubes, 1000 μL of cholesterol reagent was added. All the mixtures (salivary TC, salivary TG, salivary HDL) were incubated at 37°C for 10 minutes and the absorbance of standard and sample was measured against the blank at 505 nm in the analyser.
Salivary LDL and VLDL levels were calculated as follows:
Salivary LDL= Salivary TC-(Salivary VLDL)-Salivary (HDL)
Salivary VLDL= Salivary TG/5
Statistical Analysis
Data analysis was carried out using SPSS Software Version 20.0. Student’s unpaired t-test was done for comparing difference between means for salivary lipid levels of two study groups. Frequency tables and descriptive statistics were done. Chi-square test was done to find the association between way of tobacco consumption and salivary lipid profile in tobacco chewers. The statistical significance was accepted at p-value <0.05.
Results
The study included 15 tobacco chewers (13 males and 2 females) with a mean age of 32±6.5 years. Among different methods of tobacco consumption, about 40% were consuming arecanut with tobacco, tobacco with slaked lime (46%) and paan masala with tobacco (13.3%) respectively. The sample showed 53% tobacco chewers were chewing tobacco and 46% were placing it in buccal mucosa. The tobacco chewers would either swallow (40%) or spit (60%) the tobacco. Few tobacco chewers had no changes in oral mucosa (40%), while few reported homogenous leukoplakia (47%) and initial stages of oral sub-mucous fibrosis (13%). All the study subjects had a similar dietary pattern. (Vegetarians with occasional meat). Descriptive statistics showed that the mean±standard deviation of duration of tobacco consumption was 8±3.76 years. The frequency of daily consumption of tobacco was 3.33±1.17 times/day.
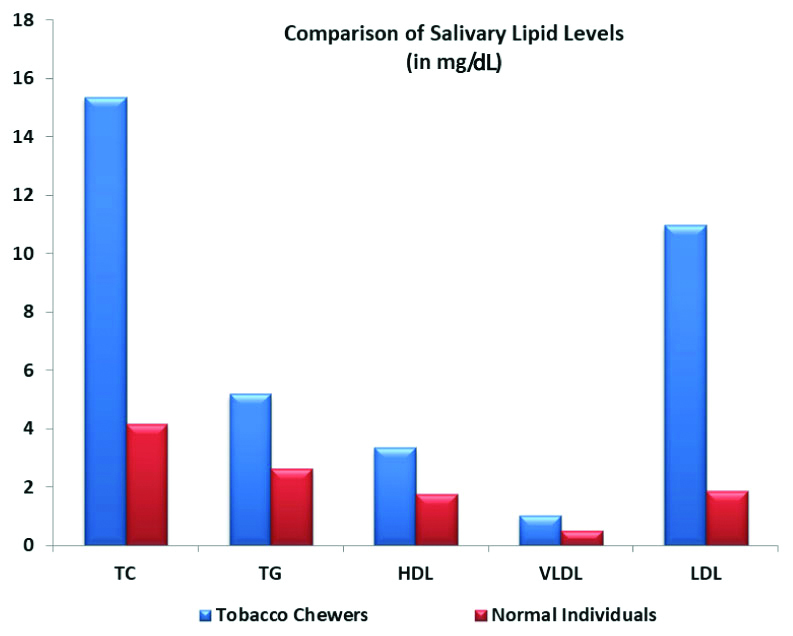
The minimum values, maximum value, mean and standard deviation values were compared for each of five parameters as a component of lipid profile for both the groups [Table/Fig-1].
Salivary lipid values in tobacco chewers and controls.
| Lipid profile in mg/dL | Mean±SD | p-value |
|---|
| Total cholesterol | Tobacco chewers | 15.37±4.82 | <0.001 |
| controls | 4.17±2.41 |
| Triglycerides | Tobacco chewers | 5.2±2.66 | 0.004 |
| controls | 2.64±1.53 |
| HDL | Tobacco chewers | 3.35±1.43 | 0.002 |
| controls | 1.78±1.02 |
| VLDL | Tobacco chewers | 1.04±0.53 | 0.004 |
| controls | 0.53±0.31 |
| LDL | Tobacco chewers | 10.97±4.28 | <0.001 |
| controls | 1.87±1.45 |
SD: Standard deviation
The mean±standard deviation in healthy individuals for salivary TC, TG, HDL, VLDL, and LDL were 4.17±2.41, 2.64±1.53, 1.78±1.02, 0.53±0.31, 1.87±1.45 (mg/dL), respectively.
In tobacco chewers the mean±standard deviation for salivary TC, TG, HDL, VLDL, and LDL were 15.37±4.82, 5.2±2.66, 3.35±1.43, 1.04±0.53, 10.97±4.28 (mg/dL), respectively. It was observed that for each of parameters of salivary lipid profile (TC, TG, HDL, VLDL and LDL), values were higher in tobacco chewers groups when compared to healthy subjects and the difference was statistically significant. (p-value <0.05) [Table/Fig-2].
Comparison between salivary lipid levels in tobacco chewers and controls.
The association between the way of tobacco consumption in tobacco chewers and salivary lipid profile showed no statistically significant difference. (p-value <0.05) [Table/Fig-3].
Association between the way of tobacco consumption in tobacco chewers and salivary lipid profile.
| Way of tobacco consumption | Salivary lipid profile (Chi-square: p-value) |
|---|
| Cholesterol | Triglycerides | HDL | VLDL | LDL |
|---|
| Arecanut with tobacco | 0.387 | 0.268 | 0.714 | 0.268 | 0.363 |
| Tobacco with slaked lime |
| Paan masala with tobacco |
Discussion
Alkaloid nicotine present in tobacco is the most addictive constituent, which is a stimulant and may develop tolerance and dependence in the users. Nicotine also shows a considerable influence on the level of lipids in the blood [1,4,5]. Lipid profile is most commonly analysed by means of cellular and chemical constituents of blood/plasma [3,16,18].
Saliva offers distinct advantages in that it implies a decreased risk of infection to laboratory staff compared to the handling of blood samples [20]. Also, because lipids are secreted in saliva, it can be used for evaluation of lipid profile [12].
Different methods have been used to analyse the lipid composition in saliva. Mixed or whole saliva is obtained by expelling the fluid directly into a collector tube, whereas, to obtain gland specific saliva, cotton swabs, absorption with small strips of filter paper or aspiration through cannulas located in the excretory duct are required. Likewise, some authors use stimulated or unstimulated saliva. The stimulation cause changes in the sample volume and in the electrolytes amount, thus altering the pH, although, they do not significantly alter the other endogenous components such as lipids and proteins [11,20]. So, in the present study, unstimulated saliva was collected using the spitting method.
The presence of lipids have been known since Doubleday (1909) and also evaluated by various authors [15,21]. A large portion of salivary lipids are associated with proteins, especially high molecular weight glycoproteins (i.e., mucins) and Proline Rich Proteins (PRPs) glycolipids, free fatty acids, phospholipids, and cholesterol. Several membranes act as sources of lipid, such as secretory vesicles, microsomes, lipid rafts and other plasma and intracellular membrane fragments of lysed cells and bacteria. In whole saliva, lipids are derived from gingival crevicular fluid outflow. Since saliva consist of a lower percentage of phospholipids, salivary lipids are not primarily of membrane origin [12,14,22]. In the present study, salivary analysis was done using whole saliva.
Lipids in saliva originate from serum element transudation, exfoliative cells and the glandular secretion. Salivary lipids are mostly glandular in origin, but some believe that they diffuse directly from serum [15]. It is known that total lipid fraction in saliva consists of neutral lipids and polar lipids. Also, under normal conditions, their values in saliva and blood may reflect the dietary pattern, as they are affected by the quality and quantity of fats consumed in the diet [20,21]. Keeping these factors in mind, the study samples were given detailed information about the collection protocol.
An association of serum lipid profile and periodontitis has been postulated by Kalburgi V et al., Losche W et al., and Iacopino AM and Cutler CW, but with contrasting results [15,23,24]. Although, these studies do not depict a strong correlation between serum lipid levels and periodontitis. Few authors debate that, a mere existence of an association between periodontitis and serum lipid levels does not establish whether periodontal disease cause increased lipid levels [23,24]. Chronic periodontitis was also found associated with salivary lipids [15]. Thus, in the present study such patients were excluded from the study sample.
Brischetto CS et al. put forward the mechanism of alterations in lipid profile linked to tobacco use. The release of adrenaline from the adrenal cortex is stimulated by nicotine, which further activates adenyl cyclase of adipose tissue. Thus resulting in lipolysis of stored TG and release of Free Fatty Acids (FFA’s). The plasma albumin binds to the released FFA’s and is transported to various parts of the body specially to the liver wherein hepatic synthesis is stimulated and cholestrol and VLDL are secreted, hence increased TG. A decrease in plasma HDL levels and increase in plasma TG and VLDL could be attributed to increase in the level of plasma FFAs [5,17,25-27].
In the present study we analysed and compared the effects of tobacco chewing on the lipid profile in tobacco chewers and the control group. The mean value of the lipid components (TC, TG, HDL, LDL, and VLDL) were increased in tobacco chewers as compared to the controls which is in accordance with studies conducted in serum samples [3,5,16,17,19,28].
HDL prevents lipid peroxidation on the cell membranes by counter balancing the oxidative damage caused by LDL. It has been suggested that HDL prevents both enzymatic and non-enzymatic generation of free radicals and thus acts as an anti-carcinogen and a powerful antioxidant [19,29]. While some authors have pointed a decrease in HDL, according to the results obtained in the present study, the HDL levels tend to increase in tobacco chewers which is in accordance with Li G et al., [1,3,5,12,18,19,28]. These findings suggest that tobacco use produces dyslipidemia. It can be an early indication towards progression to malignancy [1,4,5,9,19].
Singh S et al., and Alagendran S et al., suggested that saliva may be used for TC, TG, HDL, VLDL and to some extent for LDL monitoring, as these follow the course of plasma lipid profile and show a positive correlation [12,13]. Al-Rawi NH suggested that lipid fractions particularly TG can be assessed in saliva and may be used alone or in combination with other lipid parameters for monitoring disease activity in ischemic stroke and diabetes mellitus [14,22]. The present study also confirms that salivary lipid profile can be used to assess the derangement of lipid profile in tobacco chewers.
Singh S et al., and Karjalainen S et al., found correlation of salivary and serum cholesterol concentrations and concluded that saliva cholesterol concentrations reflect serum concentrations to some extent and thus, in the present study the increase in the salivary lipid profile could be used as a new diagnostic tool to assess the derangement of lipid profile that can used be as a diagnostic test for assessing the early sign of progression to malignancy in tobacco chewers [12,30].
Limitation(s)
The present study is a pilot study done with a limited sample to evaluate whether there are any changes in salivary lipid profile between the tobacco chewers and controls. Future research including a larger sample size would be recommended to suggest replacing the serum lipid profile with salivary lipid profile.
Conclusion(s)
Thus, saliva can be used as a non-invasive diagnostic tool for assessing lipid profile. However, a salivary diagnostic test could serve as a surrogate to replace a more conventional one, only when its diagnostic capacity is determined in terms of sensitivity, specificity, correlation with established disease diagnostic criteria and reproducibility.
SD: Standard deviation