Psoriasis is a chronic, inflammatory papulosquamous cutaneous disorder and both genetic and environmental factors play a major role in its aetiopathogenesis [1,2]. The lesions typically present as erythematous scaly well defined indurated plaques involving the extensor surfaces of the extremities and scalp [3,4]. Clinically, based on the site of involvement, psoriasis can be classified into chronic plaque psoriasis, mucosal psoriasis, erythrodermic psoriasis, nail psoriasis, guttate psoriasis, sebopsoriasis, flexural psoriasis, pustular psoriasis and palmoplantar psoriasis [5,6]. Psoriasis can be diagnosed on the clinical appearance of the lesions and biopsy if required. Severity and extent of the disease can be evaluated using the PASI score [7-9]. The main aim in the treating psoriasis is to increase the initial and rapid control of the disease process, and to sustain long-term remission [10,11]. Topical therapy is the first line of management for psoriasis. Systemic therapy comprises of photochemotherapy, conventional systemic and biological agents [2,12,13].
In developing countries like India, methotrexate is a cost-effective antipsoriatic agent given for chronic plaque type psoriasis, erythrodermic, pustular and severe psoriatic arthropathy [14]. It is still an effective treatment option with good tolerance particularly in poor countries [14].
Based on data available in literature, methotrexate is found to reduce the psoriasis severity in approximately 75% patients [15]. Extensive psoriasis in general does not respond well to the topical therapy. Other modalities of treatment like phototherapy accessible in our country and other systemic therapy either high-priced or have significant adverse effects. The rationale of the present study was to obtain the efficacy of methotrexate, if so methotrexate can be used as a cost effective systemic therapy in patients.
Materials and Methods
The current hospital based retrospective study was conducted on 86 psoriasis patients attending the Dermatology OPD at Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai, India during the study period from January 2015 to December 2017. Ethical clearance was obtained from the Institutional Ethics Committee (CSP-MED/18/FEB/42/63).
A detailed data collection for the patients was specifically designed that included demographics, clinical history, dermatological examination, laboratory investigation (initial and recent last parameters), provisional diagnosis, cumulative dose of methotrexate and other medication profile. Pre-treatment workup of all patients included complete blood count, fasting lipid profile, Blood sugars, renal and liver function tests, TB screening (chest X-ray, Mantoux test), serological tests (in selected individuals).
The study includes patients with moderate, severe, pustular, nail, erythrodermic psoriasis of both sexes aged 18-60 years. Patients with hepatic ailments, renal ailments, alcoholic abuse, hepatotoxic and nephrotoxic drug intake, pregnant and breast feeding woman, woman of reproductive age, patient suffering from the tuberculosis, congestive cardiac failure, uncontrolled hypertension were excluded from the study.
Methotrexate was started as once in a week oral or intramuscular dose of 5-10 mg (maximum upto 20 mg) and continued until there was improvement of the skin lesions clinically, following which the drug was tapered to the lowest possible maintenance dose. Additionally, folic acid 5 mg once daily was prescribed on the days when methotrexate was not prescribed in order to minimise the toxicity of methotrexate. The response to treatment for all the patients was evaluated using PASI score at the time of visit and then every month till complete resolution of the lesions. Score ranges from 0-72. For each region, the severity of erythaema, scaling and thickness was determined and graded on a scale of 0 to 4 as:
0-None, 1-Mild, 2-Moderate, 3-Severe, 4- Very severe.
The formula for calculating PASI score is as follows:
PASI score=0.2 (Eh+Ih+Dh) ×Ah+0.2 (Eu+Iu+Du) × Au+0.3 (Et+It+Dt) ×At+0.4 (El+Il+Dl) ×Al
Where, E- Erythaema, I- Induration, D- Desquamation
Ah- area of head involved in psoriasis, Au - area of upper limb, At - area of trunk, Al - area of lower limb.
Calculation of the area involved by psoriasis as follows:
1=<10%
2=10-29%
3=30-49%
4=50-69%
5=70-89%
6=≥90%
The grouping of the patients was done based on the PASI score:
PASI <10 suggests mild to moderate psoriasis
PASI between 10-20 suggests moderate to severe psoriasis
PASI >20 suggests severe psoriasis [9].
Depending on the grade of severity, methotrexate can be initiated as a single regimen or in combination with other drugs. It can be administered orally, subcutaneously or intramuscularly. A test dose of 2.5 to 5.0 mg per week has to be given and monitored, as some patients may be unduly sensitive to the drug, as per the guidelines recommended by The British and American Association of Dermatology. Following this, the dosage maybe gradually increased up to 30 mg/week depending upon the clinical response under careful monitoring [16]. Overdosing can result in methotrexate toxicity and can be potentially fatal. There are different types of toxicities which include bone marrow suppression, oral ulceration, gastrointestinal disorders, hepatotoxicity, haematological, pulmonary and neurological toxicity, amongst which hepatotoxicity has been regarded to be the most frequent limitation of methotrexate treatment. Hence, it is mandatory to supplememt the patient with folic acid as it reduces the toxicity without compromising the efficacy of treatment [17].
Statistical Analysis
Statistical analysis were done using SPSS version 20.0. Descriptive statistics like mean±standard deviation were calculated for quantitative variables and qualitative variables were represented in terms of frequency and percentage. Mean values were compared across the groups by means of t-test. Differences were considered statistically significant if p-value was <0.05.
Results
Sex Distribution
In the present study, there were a total of 86 patients, out of which 56 (65%) were male and 30 (35%) were female with mean age being 40.73±5.353 years.
In this study, PASI scoring was highest in patients with moderate to severe psoriasis with 61.6% followed by mild to moderate psoriasis (19.8%) [Table/Fig-1].
Severity assessment using PASI scoring scale.
| Scoring | Severity | Frequency (n=86) | Percentage |
|---|
| PASI <10 | Mild to moderate | 17 | 19.8 % |
| PASI 10-20 | Moderate to severe | 53 | 61.6 % |
| PASI >20 | Severe | 16 | 18.6 % |
During the initition of baseline therapy, all 86 patients were started methotrexate with a test dose of 5 mg/week. With monitoring lab profile/values normal, dose was increased according to the PASI score grading. During the first month of treatment, 53 patients were continued with 10 mg methotrexate. Since 15 patients showed poor clinical outcome, the dose was increased to upto 15 mg. In the second month, dose of six patients was increased from 10 mg to 15 mg and for four patient’s from 10 mg to 20 mg due to poor clinical response [Table/Fig-2].
Dose distribution of methotrexate in patients.
| Phase of therapy | Dosage of Methotrexate |
|---|
| 5 mg | 7.5 mg | 10 mg | 15 mg | 20 mg |
|---|
| Baseline | 86 | 0 | 0 | 0 | 0 |
| 1st month | 0 | 18 | 53 | 15 | 0 |
| 2nd month | 0 | 18 | 58 | 6 | 4 |
| 3rd month | 34 | 16 | 33 | 3 | 0 |
| 4th month | 54 | 2 | 30 | 0 | 0 |
| 5th month | 68 | 2 | 16 | 0 | 0 |
The study group included 68 patients who had chronic plaque psoriasis (79.06%), 14 patients had palmoplantar psoriasis (16.23%), 2 patients each with nail psoriasis (2.3%) and erythrodermic psoriasis (2.3%) respectively.
Baseline values of PASI score was 18.12±10.074 for psoriasis patients. PASI score at 5th month was found to be 5.50±1.97 indicating a statistically significant improvement (p<0.05) in PASI score [Table/Fig-3].
PASI 50 and PASI 75 are universally accepted and significant parameter for assessment of severity of psoriasis. Clinical outcome based on PASI score are categorized into three groups as follows. Thirteen (15.11%) patients achieved PASI response <PASI 50; 58 (67.44%) patients between PASI 50-PASI 75 and 15 patients (17.44%) >PASI 75. In this study, PASI score was evaluated on a monthly basis inorder to assess the efficacy of the therapy. Results revealed the decrease in severity of psoriasis and efficacy of the treatment.
Evaluation of outcome of psoariatic patient’s month wise using PASI Score.
| PASI score (month wise) | Mean±SD |
|---|
| Baseline | 18.12±10.07 |
| 1st month | 15.51±7.04 |
| 2nd month | 14.64±5.62 |
| 3rd month | 8.36±2.61 |
| 4th month | 6.58±1.90 |
| 5th month | 5.50±1.97 |
Gastointestinal upset was the most common adverse effect which was noted in 9 (10.5%) patients [Table/Fig-4].
| Adverse effects | No of patients |
|---|
| GI upset | 9 (10.5%) |
| Elevated liver enzymes | 7 (8.1%) |
| Leucopenia | 1 (1.2%) |
| Thrombocytopenia | 1 (1.2%) |
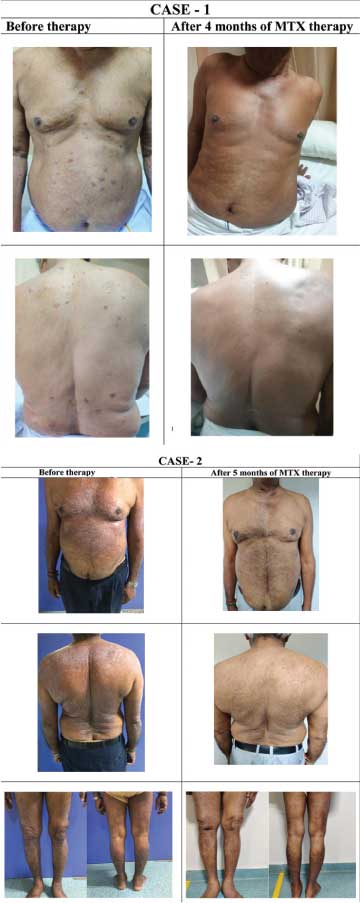
The pre and post treatment clinical photographs of the patients showing remission of the lesions after four and five months is shown in [Table/Fig-5].
Pre and post treatment clinical photographs.
Discussion
Psoriasis is a chronic inflammatory papulosquamous disorder which can lead to significant morbidity, affecting patient’s quality of life. Hence it is important to initiate the treatment at the earliest in patients with moderate to severe disease, who may require systemic therapy. Methotrexate acts by inhibiting dihydrofolate reductase and thymidylate synthase enzymes, thereby depleting the intracellular reserve of fully reduced folates which inturn affects transmethylation reactions and inhibition of DNA synthesis induces apoptosis in keratinocytes. This causes inhibition of replication and function of T and B lymphocytes that interferes with epidermal cell kinetics [18-20]. It weakens the complement C5a-induced skin response and leukotriene B4-induced intraepidermal penetration of granulocytes [21]. Also, it significantly decreases interleukin-22 levels, a cytokine that promotes inflammation of kertainocytes and inflammation of dermis [22]. Also, it induces the release of adenosine by inhibiting 5-Aminoimidazole-4 Carboxamide Ribonucleoside Transformylase (AICART), thereby playing a role of anti-inflammatory and immune modulatory agent [23].
In the present study, we included a total of 86 patients, out of which 56 (65%) were males and 30 (35%) were females which indicates a strong preponderance towards male patients which was in concordance with Karn D et al., Haider S et al., and Zhai Z et al., study [24-26]. The age group included in the study was from 18-60 years and the mean age was found to be 40.73±5.353 years. These findings were similar to Haider S et al., study (40.0±12.6) and Zhai Z et al., study (42.1±12.4) [25,26].
Baseline values of PASI score was 18.12±10.074 for psoriasis patients. This was higher when compared to Haider S et al., study (14.8±4.2)., Subrahmaian VT et al., (14.90±1.100), Opmeer BC et al., (13.4±3.6) [26-28]. In the present study PASI score at the 5th month was found to be 5.50±1.97 which showed a statistically significant improvement in the psoriatic lesions. These findings were similar to Subrahmanian VT et al., study which showed a slightly higher mean PASI of 8.00±0.200 at the end of 20 weeks and in contrast with Opmeer BC et al., which showed 5.0±4.5 at 16 weeks and Haider S et al., study with 4.9±4.3 at 8 weeks of treatment. This discrepancy could be due to the higher baseline PASI in this study compared to the aforementioned studies and hence slightly higher duration for clearance of lesions [27,28]. In this study, we evaluated PASI score on a monthly basis in order to assess the efficacy of the therapy. Majority of the patients showed a PASI of >50 (67.44%) followed by PASI >75 (17.44%) indicating a decrease in severity of psoriasis and efficacy of the treatment. The results were comparable with Subrahmaian VT et al., study with PASI >50 in 58.70% patients [27].
In this study, the most common adverse effect encountered was GI upset (10.5%). These findings were similar to Shabeer D et al., study (11.74%) and Lajevardi V et al., (13.63%) [29,30]. Elevated liver enzymes were observed when mean cumulative dose of methotrexate exceeded more than 1500 mg over a mean period of 104 weeks. This was in concordance with Haustein UF and Rytter M, study where a low dose methotrexate was used and not in concordance with Saurat JH et al., study as more adverse effects were reported due to the fact that higher dose was given to the patients without a test dose [31,32]. There were no significant drug interactions observed in medication profile. Since folic acid supplementation is mandatory, patients were supplemented with 5 mg/day except on the day of MTX inorder to minimise the GI side effects and this study also supports the above findings.
Although there are many biologicals available in the market and they show promising results, however MTX is preferred because of its cost-effectiveness.
Limitation(s)
However, limitations of our study were small sample size, retrospective nature of the study and investigations to rule out liver toxicities like procollagen III amino-terminal propeptide level, liver scan and biopsy were not carried out.
Conclusion(s)
In this study, methotrexate at a low dose less than 15-20 mg/week was an effective treatment for moderate to severe forms of psoriasis. This reduces severe adverse effects even when patients are on long term treatment. Drug interactions must be avoided. In contrast to the extensive usage of MTX at higher doses in rheumtological disorders, good efficacy was seen with lower dose regimen in dermatology. The present study provides an overview for methotrexate dosing for future consenses and guidance in daily practice as studies are scrace on this subject. Methotrexate is still a near to gold standard therapy for psoriasis, inducing quick remission and delays relapse. PASI reduction was observed during each month of therapy. If given with proper monitoring will have significantly low adverse effects.