First Case of Chronic Myeloid Leukaemia Associated Microfilariasis at the Brazzaville University Hospital
Lethso Thibaut Ocko Gokaba1, Olivia Firmine Atipo Galiba2, Géril Sekangue Obili3, Lydie Ocini Ngolet4, Alexis Elira Dokekias5
1 Medical Doctor, Department of Haematology, Brazzaville University Hospital, Brazzaville, Congo.
2 Medical Doctor, Department of Clinical Haematology, Brazzaville University Hospital, Brazzaville, Congo.
3 Medical Doctor, Department of Parasitology, Brazzaville University Hospital, Brazzaville, Congo.
4 Medical Doctor, Department of Clinical Haematology, Brazzaville University Hospital, Brazzaville, Congo.
5 Medical Doctor, Department of Clinical Haematology, Brazzaville University Hospital, Brazzaville, Congo.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Lethso Thibaut Ocko Gokaba, Brazzaville Univerty Hospitol BP: 32, Brazzaville, Congo.
E-mail: ockthib@yahoo.fr
The association between Chronic Myeloid Leukaemia (CML)-Filariasis is rare. Moreover, finding microfilariae in the bone marrow is uncommon. We reported the case of 47-year-old patient living in a rural area who was admitted in the haematology ward in order to further examine a splenomegaly he had which was associated with a leucocytosis. Physical examination revealed oedematous lower limbs and peripheral blood film comment showed the presence of myelocytosis and eosinophilia. The myelogram showed hyperplasia of immature granulocytes and presence of Loa loa, Wuchereria bancrofti and Mansonella perstans microfilariae. The diagnosis of CML was then confirmed by the presence of the gene translocation t (9; 22) and the MBCR-ABL transcription type b3a2 on cytogenetic examination. This case represents a semiotic interest because oedema of both upper and lower limbs associated with a leucocytosis in filarial endemic zone must first of all indicate a haematological malignancy associated with filariasis.
Blood smear, Bone marrow smear, Malignant homeopathy, Polyparasitism
Case Report
A 47-year-old, male community health agent, living in a forest area in the north of the Congo, presented to the haematology department for a severe left upper abdominal pain, with a history of seven months abdominal pain and progressive fatigue. After physical examination of the patient, we found pallor, hands and ankle oedema, a hepatomegaly with smooth lower border (22 cm in right mi-clavicular line) splenomegaly but no signs of filaria. The rest of the physical examination was unremarkable and no radiographical examination was done.
Full blood count showed: leukocytes: 114,000/mm3; haemoglobin: 7.8 g/dL; haematocrit: 23.8%; MCV: 84, 4 fL, platelets: 310,000/mm3. Blood smear: blasts: 4,580/mm3; myelocytes: 8,020/mm3; metamyelocytes (mauve arrow): 27,520/mm3; neutrophils: 56,200/mm3; eosinophil: 14,910/mm3 and erythroblasts: 3,440/mm3.
Peripheral blood samples were collected in an EDTA tube at the bend of the elbow, while a sample of the bone marrow was collected by medullar aspiration and spread on object slides. Cytological examination after May Grunewald Giemsa (MGG) staining revealed:
- At low magnification (x10): The presence of isolated filiform and serpiginous elements [Table/Fig-1a,b];
- At high magnification (x100): Microfilariae [Table/Fig-1c,d] having large ovoid nuclei, present right to the thinned caudal region and absent from the long rostral space, compatible with Loa loa. Other microfilariae have a sheath, a short cephalic space, a thinned caudal extremity with small sub-terminal nuclei, in favour of Wuchereria bancrofti. Others still, had a very short cephalic space, a glove finger-like posterior extremity with terminal nuclei, without a sheath, suggesting Mansonella perstans. Parasite density was estimated to be 130,000 parasites/mm3.
a) (X10) isolated serpiginous elements. b) (X10) clustered serpiginous elements. (c) (X100) type Loa loa microfilaria (red arrow), metamyelocyte (mauve arrow). (d) (X100) type Wuschereria bancrofti microfilaria (red arrow). All slides were stained with May Grünwald Giemsa.
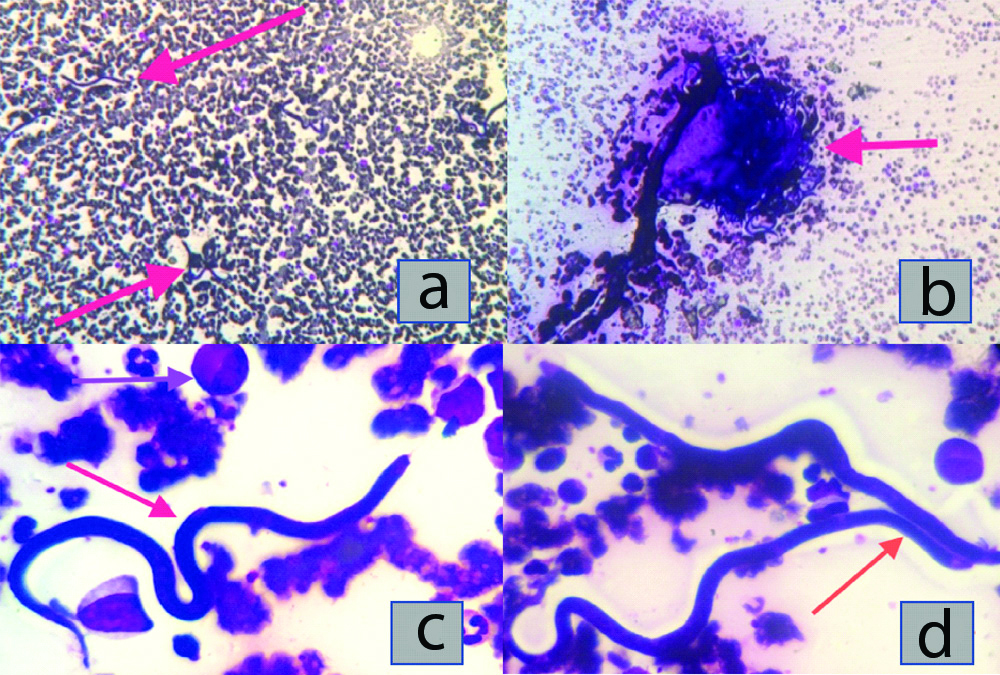
Bone marrow aspirate showed dysmegakaryopoiesis and myeloid hyperplasia without a maturation hiatus neither a blast excess. We noticed a large number of microfilariae.
Cytogenetic studies showed t (9; 22) in all cells examined and molecular studies reported transcript MBCR-ABL type b3a2 MBCR-ABL. The diagnosis of CML in its chronic phase associated with circulating filariasis was made.
Imatinib was initiated at the dosage of 400 mg by oral route daily and ivermectin 4 tablets of 200 μg each in a progressively increasing dose until the total dose of 4 tablets is reached.
The patient achieved normal haematological response (CBC back to normal, neither enlarged spleen nor liver) within three weeks. The patient was released 24 days after his admission and never came back for follow-up.
Discussion
CML and filariasis are groups of conditions characterised by a chronic malignant myeloid proliferation associated with the presence of microfilariae. CML is a myeloproliferative syndrome linked to the Philadelphia chromosome (Ph.) and/or BCR-ABL gene while filariasis is transmitted through insect bites. The female insect transmits the microfilariae, which will then be found according to the species in the blood (occult microfilariae) or the skin (subcutaneous). Rarely reported, the coexistence of these conditions found on cytological examination, is not described in African literature but is reported in India [1]. We reported an incidental discovery in the haematology laboratory of the Brazzaville University Hospital Center.
The association CML-filariasis is rarely reported in literature. However, several authors have reported it [1,2]. Filariasis are endemic conditions in tropical and sub-tropical regions of Asia, Central Africa and South America, loasis being only found in Central Africa [3,4]. CML is more observed in Brazzaville [5]. The discovery of three types of microfilariae during CML seems to be a coincidental because other associations have been reported especially with solid tumours and malignant homeopathies like Type B acute lymphoblastic leukaemia, Hodgkin lymphoma and Type 4 eosinophilic acute myeloid leukaemia [1,4].
Lower limbs oedema is observed in lymphatic filariasis often caused by Wuchereria bancrofti and those of the hands in loasis, named calabar oedema [3], as described in our case. The cases described in literature were asymptomatic. Also, hyper eosinophilia, frequently observed in filariasis [6], was found in this patient. Kinger M, underlines the absence of eosinophilia in his case. According to the author, this should be as a result of the oxidative stress capable of altering the immune response [2]. Circulating erythroblastosis, unusually described in CML could be explained by the myelofibrosis but could also be a consequence of hyperparasitaemia associated to a polyparasitic marrow. The microfilaria species associated with CML and other malignant diseases could be dependent either on the geographical distribution of filariasis in endemic areas, with Wuchereria bancrofti being the most commonly reported species of malignant homeopathies [2]; or on accidental passage of microfilariae into the bone marrow; either to susceptibility to filarial infections induced by immunosuppression caused by malignant homeopathy; or to decreased immunosurveillance observed in chronic parasitic diseases and antigenic stimulation promoting immune cell proliferation in the case of parasitosis [7].
Conclusion(s)
The coexistence of microfilariae and CML is a coincidence. It is also reported together with other tumours. The species of microfilariae are the same except for Loa loa, observed specifically in central Africa.
Author Declaration:
Financial or Other Competing Interests: No
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Aug 26, 2019
Manual Googling: Nov 27, 2019
iThenticate Software: Dec 18, 2019 (8%)
[1]. Pahwa S, Saksena A, Singh A, Daga MK, Singh T, Blastic phase of CML with microfilaria: A rare case reportJ Clin Diagn Res 2015 9(1):ED09-10.10.7860/JCDR/2014/10545.542425737999 [Google Scholar] [CrossRef] [PubMed]
[2]. Kinger M, Chakrabarti PR, Surabhi S, Kiyawat P, Unusual case of bancrofti filariasis co-existing with chronic myeloid leukemiaAnn Trop Med Public Health 2014 7:64-66.10.4103/1755-6783.145028 [Google Scholar] [CrossRef]
[3]. Arundhati AK, Kumar R, Acute lymphoblastic laekemia with microfilaria: A rare coincidence in bone marrow aspirateIndian J Hematol Blood Transfus 2011 27:111-12.10.1007/s12288-011-0066-222654304 [Google Scholar] [CrossRef] [PubMed]
[4]. Jain S, Sharma M, Jasmita Tyagi S, Common parasite with uncommon associationMediterr J Hematol Infect Dis 2011 3(1):01-05.10.4084/mjhid.2011.01521625318 [Google Scholar] [CrossRef] [PubMed]
[5]. Ngolet LO, Kocko I, Galibaatipo Tsiba FO, Guelongo Okouango Ova JD, Elira Dokekias A, Imatinib mesylate in chronic myelogenous leukaemia: A Congolese experienceEAMJ 2006 93:118-22. [Google Scholar]
[6]. Anane S, Les étiologies parasitaires d’une hyperéosinophilie sanguineAnn Biol Clin 2006 64(3):219-29. [Google Scholar]
[7]. Kerketta LS, Ghosh K, Circulating microfilariae in haematological malignancies: do they have a role in pathogenesis?J Helminthol 2018 92(1):125-27.10.1017/S0022149X1700006228181472 [Google Scholar] [CrossRef] [PubMed]