Family planning and fertility regulation using modern methods of contraception had been identified as an effective means of controlling the problem of unwanted pregnancy and unsafe abortion [1]. Statistics indicated that, despite the high rate of sexual activity among the young and married women of reproductive age, the current prevalence rate for contraceptive use, 11%-13%, in Nigeria, and many developing countries, is extremely low [2]. Consequently, many unmet needs for contraceptive use include unintended pregnancies and illegal abortions ranging from 18.3% to 64.0% of cases [3]. While female contraceptive methods work differently and affect the quality of vaginal microbial flora both in different population and species variation, hormonal contraceptives such as oestrogen and progestogen pills, depo progestin injection, vaginal ring and hormonal IUD prevent pregnancy by interfering with ovulation and suppress the release of an egg from the ovaries [4]. The methods such as condoms or the diaphragm act as a barrier to reduce the possibility of sperm and egg meeting [5,6] while steroid-based IUDs and oral pills may affect the physiology and host immune responses thereby exposing women to risks of reproductive tract infections [7].
With the current increase in the level of awareness on reproductive health globally, development and introduction of modern contraceptives, establishment of organised family planning and the desire of families to regulate their family sizes for a more healthy life, more women are increasingly taking to various contraceptive use [8,9]. However, Nigerian National Demographic and health Survey data indicated that only about 15% of sexually active Nigerian women practice effective contraception [10,11].
Vagina is crucial in sexual and reproductive health. While healthy vagina is colonised by mutually symbiotic flora of the genus Lactobacillus protecting host from disease and maintaining the acidity of the vaginal pH [12], a disruption of vaginal flora can result in a change in its ecology [13]. While the use of antimicrobial agents, high estrogen content of oral contraceptive and spermicide, alter the pH of the vagina which then alters the ecology of the vaginal flora [14], the use of IUDs can result in production of inflammation and changes in the vaginal epithelium [15] to expose women to microbial infections [16]. Consequently, candidiasis, one of the mucous opportunistic infections of the genital system, may occur as a result of an increase in the level of glycogen which may be a good source of carbon for Candida species to survive and accelerate multiplication of yeast and pseudohyphae [17].
Since vagina is a complicated environment and the use of contraceptives by women is inevitable, certain contraceptive methods have been reported to increase host susceptibility to infections [18] and cause several contraceptive-use related diseases [19]. While candidiasis, Trichomonas vaginalis and Chlamydia trachomatis have been found among oral contraceptive users [14], pelvic inflammatory diseases and reproductive tract infections have been associated with prolonged use of IUDs and other contraceptives [20]. Despite some reports on infectious diseases associated with the use of different contraceptives, there is a dearth of information on the distribution and susceptibility of microbial flora associated with asymptomatic women using different contraceptive methods- oral contraceptive pills [21], intrauterine contraceptive device [22,23], hormonal contraceptive injections [24] and injectables [25] in South West Nigeria.
This study was, therefore, aimed at investigating the microbial flora and antimicrobial susceptibility of microorganisms isolated from vagina of asymptomatic women using different contraceptive methods.
Materials and Methods
Collection Centres
Three family planning centres including family planning clinics at Olabisi Onabanjo University Teaching Hospital, Centre for Research in Reproductive Health and Owokoniran Memorial Hospital in Sagamu, Ogun State, Nigeria, were used for sample collections between June and December, 2017. These centres were selected because they constitute major centers for reproductive health hospitals attending to multi-ethnic groups in the South-western part of Nigeria.
The ethical approval to carry out this prospective study was granted with certificate number OOU/TH/DA 126/250 by Research and Ethics Review Committee of Olabisi Onabanjo Teaching Hospital, Sagamu, Ogun State, Nigeria.
All volunteer women who were presently following one of the contraceptive methods, sexually active, ages between 20 and 45 years and were not presently on any antibiotics or having history of antibiotics use three weeks prior the sampling periods were selected. Women not using specific contraceptive devices were also taken up for the study as control group. The selected women were given informed consent forms and questionnaires to fill and signed before being accepted for the research purposes.
Sample Collection
High vaginal swab samples were collected. The samples were taken from the cervical canal with sterile cotton swab after cleaning the vaginal area with sterile water and inserting moistened sterile speculum into the cervix. The cotton swabs were gently rotated against the vaginal wall to obtain specimens which were aseptically transferred into the holder, given a numerical labelling before being transferred to the laboratory within two hours of collection for processing.
Direct smear gram stain:
Direct smear of the swab samples were prepared on clean glass slides, allowed to air dry and Gram stained using modified Christian Ham Gram staining method [19] before being examined using oil immersion objective lens (X100) with the condenser iris diaphragm being opened sufficiently to give good contrast.
Wet preparation using normal saline
The vaginal smears were prepared on clean grease free glass slides and a drop of normal saline was added to each smear, mixed thoroughly and covered with cover glass. The prepared wet smear slides were examined using X10 and X40 objective lens for detection of abnormal cells such as pus cells, white blood cells, epithelia, yeast cells and protozoa before being quantified per high power field.
Detection of yeast cells using potassium hydroxide solution
A drop of 30% potassium hydroxide solution was added to vaginal smear on glass slides, mixed and covered with slips. The glass slides were examined using X10 and X40 objective lens for detection of yeast cells. The cells showed oval budding with pseudo-hyphae which was quantified per field viewed.
Bacteriological Examination of Samples
The vaginal swab samples was aseptically streaked on three different culture media including MacConkey agar (LAB M), Chocolate agar containing 10% human blood cells (LAB M) and Sabouraud Dextrose Agar (LAB M). The different agars were prepared according to the manufacturer’s instructions following good standard laboratory operating procedures. The prepared agar surface were dried at 45°C for 15 minutes prior to use after which the vaginal swab samples were aseptically streaked onto the different agar plates which were labeled according to the numerical identification numbers earlier assigned to swab samples. All the MacConkey agar plates were incubated in an inverted position at 37°C for 24 hours to produce observable growth colonies. Also, all chocolate agar plates were incubated in carbon-dioxide jar at 37°C for 24 hours. Further 24 hours incubation period was allowed for the plates without growth within 24 hours before final results were recorded. Sabouraud dextrose agar plates were incubated at 30°C for 48 hour. Observable colonies on agar plates were subjected to series of bacteriological and biochemical tests performed according to Murray P et al., [26].
Antimicrobial susceptibility testing using disc diffusion method (Kirby-Bauer)
The bacterial inocula was prepared in 1% sterile peptone water and incubated for 2 hours at 37°C to produce a slight turbidity that was compared with 0.5 McFarland standards. The adjusted inocula were made into lawn with sterile cotton swabs on dried Mueller-Hinton agar surface. The agar surface was allowed to dry for 15 min before a commercially-prepared Gram negative multi disc containing ciprofloxacin (5 μg), ofloxacin (5 μg), ceptazidime (30 μg), nitrofurantoin (300 μg), cefuroxime (30 μg), gentamycin (10 μg), amoxicillin/clavulanate (30 μg) and ampicillin (10 μg) was aseptically placed on plates containing Gram negative bacteria while Gram positive multi-disc containing erythromycin (30 μg), cloxacillin (5 μg), gentamycin (10 μg), ceftazidime (30 μg), augmentin (10 μg), streptomycin (10 μg), ciprofloxacin (5 μg) and tetracycline (10 μg) was aseptically placed on plates containing Gram positive bacteria. The assay was done in duplicate before incubating at 37°C for 24 hours. The inhibition zones formed around each disc were measured and recorded according to CLSI while the average from duplicate readings was recorded [27].
Candida Susceptibility Testing
Candida species were prepared by inoculating yeast extract broth and incubating for 2 hours at 30°C to produce a slight turbidity that was compared to 0.5 McFarland standards. One hundred microlitre (100 μL) of the standardised inocula were dispensed on the surface of the dried Sabouraud dextrose agar plates and spread evenly with sterile cotton swab. The cultures were allowed to stand for 30 min. Agar wells were made with heat sterilised 6 mm cork borer and different antifungal-agents including ketoconazole (100 μg/mL), fluconazole (100 μg/mL), clotrimazole (100 μg/mL), nystatin (100 UI), griseofulvin (100 μg/mL) while commercially prepared amphotericin B (20 μg) discs were introduced to the seeded plates. The discs were gently pressed down to ensure full contacts. All the plates were labeled and incubated in an inverted position at 30°C for 24 hours. A clear inhibition zone around each of the disc was measured to the nearest millimetre using a metric ruler.
Determination of Minimal Inhibitory Concentrations (MICs) by macrobroth dilution method
The MICs for the five different antibacterial agents, to which the isolates were slightly or significantly susceptible, were determined in duplicate by the macrobroth dilution method in Mueller Hinton broth according to Richard S et al., [28]. Different concentrations ranging between 0.0019 and 375 μg/mL of the antibacterial agents were prepared from stock concentrations of Tetracycline, Kanamycin, Amoxicillin, Erythromycin and Ciprofloxacin in double strength Mueller Hinton broth by serial dilution before they were inoculated with 100 μL of each of the bacterial strains adjusted to 0.5% Mcfarland standard and incubated at 37°C for 24 hours. For the determination of the MICs for the antifungal agents, different concentrations ranging between 0.061 and 500 μg/mL of clotrimazole, ketoconazole and fluconazole were prepared in double strength Sabouraud dextrose agar by serial dilution before being inoculated with 100 μL of each adjusted fungal isolate and incubated at 30°C for 72 hours. Blank Mueller Hinton broth was used as negative control. The MIC was defined as the lowest dilution that showed no bacterial growth in the Mueller Hinton broth.
Determination of Minimum Bactericidal and Fungicidal Concentrations (MBC/MFC)
For the determination of the MBC and MFC according to Olajuyigbe OO and Afolayan AJ [29], the MIC tube and the next two broth cultures that showed no growth were subcultured on sterile nutrient agar and potato dextrose agar plates, while the MBC and MFC assay plates were respectively incubated for 24 hours and 72 hours, the concentration that did not produce any bacterial or fungal growth on the solid medium was regarded as MBC and MFC values for the antibiotics.
Statistical Analysis
All information collected were compiled and data were analysed using SPSS Software programme (version 18.0). Descriptive and analytical results are presented through tables and figures.
Results
The socio-demographic profiles showed ages of the 406 women in the study population were between 20-45 years (mean age 32.5 years) and 40.88% of the subjects were in 31-35 years age group. The majority of the population in the study were literates with 40% of them up to tertiary education. They included civil servants, students, business women and full time housewives who were sexually active with an average of two times sexual intercourse per week as shown in [Table/Fig-1].
Social and demographic profile of 356 study population and 50 women in control group.
| Age (Years) | 20-25 (n=38) | 26-30 (n=71) | 31-35 (n=156) | 36-40 (n=51) | 41-45 (n=40) |
|---|
| Education background |
| (a) Primary | 6 | 16 | 43 | 17 | 4 |
| (b) Secondary | 15 | 31 | 59 | 13 | 14 |
| (c) Tertiary | 17 | 24 | 54 | 21 | 22 |
| Marital status |
| (a) Single | 24 | 22 | - | - | - |
| (b) Married | 14 | 49 | 156 | 51 | 39 |
| (c) Divorcee | - | - | - | - | 1 |
| Occupation |
| (a) Trading | 19 | 29 | 83 | 21 | 18 |
| (b) Civil servant | 5 | 22 | 68 | 25 | 22 |
| (c) Housewife | 8 | 9 | 5 | 5 | - |
| (d) Student | 6 | 11 | - | - | - |
| No. of children |
| (a) 1-3 | 17 | 67 | 80 | 12 | 5 |
| (b) 4-5 | - | 4 | 47 | 16 | 19 |
| (c) 6 and above | - | - | 29 | 19 | 16 |
| (d) No child | 21 | - | - | 4 | - |
| Sexual activity per week |
| (a) 1-2 times | 23 | 24 | 84 | 34 | 25 |
| (b) 3 and above | 15 | 47 | 72 | 17 | 15 |
| Demography of 50 women in the control group |
| Age (Years) | 20-25 (n=10) | 26-30 (n=10) | 31-35 (n=10) | 36- 40 (n=10) | 41-45 (n=10) |
| Education background |
| (a) Primary | 2 | 2 | 3 | 4 | - |
| (b) Secondary | 5 | 4 | 2 | 3 | 3 |
| (c) Tertiary | 3 | 4 | 5 | 3 | 7 |
| Marital status |
| (a) Single | 5 | 4 | - | - | - |
| (b) Married | 5 | 6 | 10 | 10 | 9 |
| (c) Divorcee | - | - | - | - | 1 |
| Occupation |
| (a) Trading | 1 | 2 | 6 | 5 | 3 |
| (b) Civil Servant | 3 | 4 | 4 | 5 | 7 |
| (c) Housewife | - | 3 | - | - | - |
| (d) Student | 6 | 1 | - | - | - |
| No. of children |
| (a) 1-3 | 2 | 8 | 8 | 8 | 2 |
| (b) 4-5 | - | 2 | 1 | 1 | 4 |
| (c) 6 and above | - | - | 1 | 1 | 4 |
| (d) No child | 8 | - | - | - | - |
| Sexual activity per week |
| (a) 1-2 times | 2 | 3 | 7 | 4 | 7 |
| (b) 3 and above | 8 | 7 | 3 | 6 | 3 |
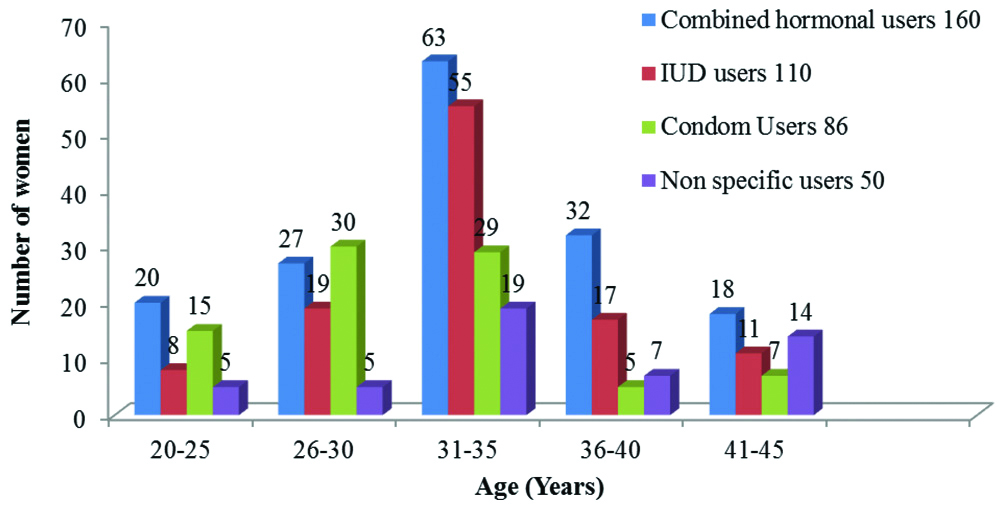
Out of 406 women sampled, 160 (39.4%) women were combined hormonal users, 110 (27.1%) women were IUD users, 86 (21.2%) women were condom users and 50 (12.3%) women with non-specific user of methods including lactation, amenorrhoea and coitus interruptus were considered as control group. Although majority of the women in each age group used combined hormonal contraceptives and the use of contraceptives by women increased with ages ranging from 20 to 35 years and decreased from 36 to 45 years, contraceptives were more used by women in ages 31-35, followed by women in ages 26-30 years while women in other studied age ranges had varied patterns of contraceptive usages as shown in [Table/Fig-2].
Distribution of contraceptive usages among women of different age groups.
Microscopic examination of the vaginal specimens in saline and potassium hydroxide solutions showed the presence of epithelial cells ranging from 3-8 cells per High Power Field (HPF) in 88.2% of the women, pus cells ranging from 2-5 cells/HPF in 37.9% of the women, yeast cells in 25.6% of the women and trophozoite stage of Trichomonas vaginalis in 1.5% women of the sampled population. While more women in ages 31-35 had epithelial cells, pus cells and yeasts, T. vaginalis was more recorded in women in ages 21-25 years as shown in [Table/Fig-3].
Result of the microscopic examination of vaginal swab samples.
| Age (Years) | Epithelia cells | Pus cells | Yeast cells | Protozoa T. vaginalis |
|---|
| 21-25 | 40 | 12 | 10 | 5 |
| 26-30 | 62 | 36 | 09 | 1 |
| 31-35 | 166 | 74 | 53 | - |
| 36-40 | 44 | 14 | 18 | - |
| 41-45 | 44 | 18 | 14 | - |
| Total | 356 | 154 | 104 | 6 |
| Control group | Epithelia cells | Pus cells | Yeast cells | Protozoa T. vaginalis |
| 21-25 | 1 | 1 | 1 | - |
| 26-30 | 2 | 2 | 1 | - |
| 31-35 | 4 | 3 | 3 | - |
| 36-40 | 2 | 2 | 2 | - |
| 41-45 | 2 | 2 | 2 | - |
| Total | 13 | 10 | 9 | |
From the bacteriological analysis of the swab samples collected, 162 pure microbial strains were identified and this gave microbial prevalence of 39.9% in the studied population. Of the 160 women using the different contraceptive methods, more strains of bacteria and fungi were isolated from women in ages 31-35 years. While more candida species were isolated from 110 women using IUDs, more bacteria strains were isolated from women using combined hormonal contraceptives. The distribution of bacterial strains isolated from the different contraceptive users (n=356) showed that 8.1% (29) women using combined hormonal contraceptives, 2.53% (9) barrier users, 1.97% (7) IUDs users and only 10% (5) of the women (n=50) in the control groups were bacteria carriers. On the contrary, while the distribution of fungal strains isolated showed that 16.01% (57) IUDs users, 5.7% (23) combined hormonal users, 5.06% (18) barrier users and 16.0% (8) of women (n=50) in the control group had fungal infection. While T. vaginalis was isolated from combined hormonal users 1.2 (5) and IUD users 0.3% (1), this organism was absent in the samples collected from other contraceptive users and non-users as shown in [Table/Fig-4]. While the bacteria isolated included Escherichia coli (11), Klebsiella oxytoca (8), Proteus mirabilis (6), Staphylococcus aureus (11) and Group B beta-haemolytic streptococci (12) and the Candida species included Candida albicans (74), Candida tropicalis (25) and Candida stellatoidea (17).
Prevalence of microbes among the contraceptive users and non users.
| No of population study | No of bacteria isolated | No of candida species isolated | No of T. vaginalis | Subjects with no growth |
|---|
| Combined hormonal | 160 (39.4%) | 29 (7.1%) | 23 (5.7%) | 05 (1.2%) | 103 (25.4%) |
| 20-25 | | 5 | 4 | 04 | |
| 26-30 | | 08 | 6 | 01 | |
| 31-35 | | 10 | 9 | - | |
| 36-40 | | 4 | 2 | - | |
| 41-45 | | 2 | 2 | - | |
| Intra uterine devices (IUD) | 110 (27.1%) | 7 (1.97%) | 57 (16.01%) | 01 (0.3%) | 45 (12.64%) |
| 20-25 | | - | 06 | 01 | |
| 26-30 | | - | 18 | - | |
| 31-35 | | 05 | 20 | - | |
| 36-40 | | 02 | 08 | - | |
| 41-45 | | - | 05 | - | |
| Barrier (Condom) | 86 (21.2%) | 9 (2.53%) | 18 (5.06%) | 0 | 59 (16.57%) |
| 20-25 | | 01 | - | - | |
| 26-30 | | 03 | 06 | | |
| 31-35 | | - | 12 | - | |
| 36-40 | | 03 | - | - | |
| 41-45 | | 02 | - | - | |
| Control (non users) | 50 (12.3%) | 05 (10.0%) | 8 (16.0%) | 0 | 37 (74.0%) |
| 20-25 | | - | - | - | |
| 26-30 | | - | 02 | - | |
| 31-35 | | 02 | 05 | - | |
| 36-40 | | - | 01 | - | |
| 41-45 | | 03 | - | - | |
The antibacterial assay showed that all the bacterial species were susceptible to ciprofloxacin, ofloxacin and nitrofurantoin while they were resistant to augmentin, ampicillin and cloxacillin as shown in [Table/Fig-5].
Percentages of susceptibility of each bacterial isolate to each of the antibiotics.
| A | B | C | A | B | C | A | B | C | A | B | C | A | B | C |
|---|
| Proteus mirabilis | Klebsiella oxytoca | Escherichia coli | Group B beta-haemolytic streptococci | Staphylococcus aureus |
|---|
| CAZ | 50% | - | 50% | 50% | - | 50% | - | 40% | 60% | - | 30% | 70% | 35% | 20% | 45% |
| CPR | 99% | - | 1% | 90% | - | 10% | 90% | - | 10% | 99% | 1% | - | 100% | - | - |
| OFL | 99% | - | 1% | 90% | - | 10% | 90% | - | 20% | 80% | 10% | 10% | 96% | 2% | 2% |
| GEN | - | 30% | 70% | - | 23% | 77% | 85% | 5% | 10% | 30% | 30% | 40% | 60% | 10% | 30% |
| KAN | - | 27% | 73% | - | 10% | 90% | 65% | 10% | 30% | 20% | 30% | 50% | 60% | 20% | 20% |
| ERY | 65% | 5% | 30% | 50% | 20% | 30 | 40% | 35% | 25% | 70% | 10% | 20% | - | 50% | 50% |
| TET | - | 20% | 80% | 55% | 10% | 35% | 30% | 5% | 65% | - | 30% | 70% | - | 45% | 55% |
| CXC | - | - | 100% | - | - | 100% | - | - | 100% | - | - | 100% | - | - | 100% |
| AUG | - | - | 100% | - | - | 100% | - | - | 100% | - | - | 100% | - | - | 100% |
| NIT | 50% | 30% | 20% | 95% | 5% | - | 96% | 4% | - | 80% | 15% | 5% | 94% | 6% | - |
| AMP | - | - | 100% | - | - | 100% | - | - | 100% | - | - | 100% | - | - | 100% |
| AMX | - | 15% | 85% | 10% | 30% | 60% | - | - | 100% | - | | 100% | 5% | 10% | 85% |
| Key | A=Susceptible (mm) | B=Intermediate susceptible (mm) | C=Resistant (mm) | Key: | A=Susceptible (mm) | B =Intermediate susceptible (mm) | C=Resistant (mm) |
| Ceftazidime (CAZ) | ≥18 | 15-17 | ≤14 | Nitrofurantoin (NIT) | ≥17 | 15-16 | ≤14 |
| Ciprofloxacin (CPR) | ≥21 | 16-20 | ≤15 | Ampicillin (AMP) | ≥17 | 14-16 | ≤13 |
| Ofloxacin (OFL) | ≥21 | 16-20 | ≤15 | Erythromycin (ERY) | ≥23 | 14-22 | ≤13 |
| Gentamicin (GEN) | ≥15 | 13-14 | ≤12 | Tetracycline (TET) | ≥19 | 15-18 | ≤14 |
| Augmentin (AUG) | ≥15 | 13-14 | ≤12 | Cloxacillin (CXC) | ≥13 | 12-10 | ≤10 |
| Kanamycin (KAN) | ≥18 | 17 | <16 | Amoxicillin (AMX) | ≥17 | 14-16 | ≤13 |
The antifungal assay showed that 80% of the fungal isolates were susceptible to ketoconazole with inhibition zones ranging between <20 and 28 mm, nystatin (75%) with inhibition zones ranging between 10 and ≥15 mm, clotrimazole (65%) with inhibition zones ranging between <11 and ≥20, fluconazole (60%) with zones of inhibition ranging between <14 and ≥19 mm while they were all resistant to griseofulvin [Table/Fig-6].
Susceptibility pattern of Candida species.
| Antifungal agents | Susceptible | Intermediate | Resistant |
|---|
| mm |
| Ketoconazole | (28) 80% | (21-27) 15% | (<20) 5% |
| Fluconazole | (≥19) 60% | (15-18) 25% | (<14) 15% |
| Clotrimazole | (≥20) 65% | (12-19) 30% | (<11) 5% |
| Nystatins | (≥15) 75% | (10-14) 15% | (No zone) 10% |
| Griseofulvin | - | - | (No zone) 100% |
The MIC and MBC obtained from five different antibacterial agents against the microbial isolates are as shown in [Table/Fig-7]. Interpreting the susceptibility of the bacterial isolates according to the MIC breakpoints recommended by BSAC [30] as ≤0.25 mg/L for erythromycin, ≤0.5 mg/L for ciprofloxacin, ≤1 mg/L for tetracycline, ≤4 mg/L for amoxicillin and ≤8 mg/L for kanamycin for Enterobacteriaceae, Staphylococcus species, Enterococcus species and Gram negative aerobes [30] high percentages of the bacterial isolates were considered resistant as their MICs were mostly above the recommended MIC breakpoints. For the antifungal activities, the MICs for Candida stellatoides ranged between 0.488 and 7.81 μg/mL for clotrimazole, 0.98 and 3.90 μg/mL for ketoconazole and between 15.63 μg/mL and 62.5 μg/mL for fluconazole. Those of Candida tropicalis ranged between 0.98 μg/mL and 7.81 μg/mL for fluconazole, between 0.488 μg/mL and 3.90 μg/mL for ketoconazole and between 1.96 μg/mL and 7.81 μg/mL for fluconazole. Those of Candida albicans ranged between 0.98 μg/mL and 31.25 μg/mL for clotrimazole, between 0.488 μg/mL and 7.81 μg/mL for ketoconazole and between 0.98 μg/mL and 62.5 μg/mL for fluconazole. The minimum fungicidal concentrations were equal to the MICs or 1-2 folds greater than the MICs.
Minimum Inhibitory (MICs), bactericidal (MBCs) and fungicidal (MFCs) concentrations of the isolated microorganisms.
| | Susceptibility of isolates from women in the sampled population |
|---|
| | Minimum inhibitory concentrations (MICs) | Minimum bactericidal concentrations (MBCs) |
|---|
| Organisms | No of strains | Cip | Amx | Ery | Tet | Kan | Cip | Amx | Ery | Tet | Kan |
|---|
| | μg/mL | μg/mL |
| Staphylococcus aureus | 11 | 0.975-7.812 | 0.586->300 | 0.976-125 | 43.75-350 | 23.43->375 | 1.95-7.812 | 1.72->300 | 1.95-250 | 87.5-350 | 46.87-375 |
| Proteusmirabilis | 06 | 7.812-62.25 | 37.5-> 300 | 62.5-250 | 350 ->350 | 46.87->375 | 15.625-31.25 | 75-300 | 125-250 | 350 | 93.75-375 |
| Escherichiacoli | 11 | 0.22-15.625 | >300 | 31.25-250 | 43.75-350 | 11.72-375 | 0.44-15.625 | >300 | 62.5-250 | 21.88-350 | 23.43-375 |
| Klebsiellaoxytoca | 8 | 0.488-15.63 | 0.586-300 | >250 | 87.5->350 | 46.87->375 | 0.976-31.25 | 4.49-300 | >250 | 43.75-350 | 93.75-375 |
| Group B beta-haemolytic streptococci | 12 | 1.95-31.25 | 9.376-300 | 31.25->250 | 43.75->350 | 93.74->375 | 3.9-62.5 | 18.75->300 | 62.5->250 | 87.5-350 | 93.75->375 |
| | Minimum Inhibitory Concentrations (MICs) | Minimum Fungicidal Concentrations (MFCs) |
| | Clot | Ket | Fluc | | | Clot | Ket | Fluc | | |
| | μg/mL | | | μg/mL | | |
| Candidastellatoides | 17 | 0.488-7.81 | 0.98-3.90 | 15.63-62.5 | | | 1.96-31.25 | 0.97-15.63 | 31.25-125 | | |
| Candidatropicalis | 25 | 0.98-7.81 | 0.488-3.90 | 1.96-7.81 | | | 1.96-62.5 | 0.96-15.63 | 3.96-31.25 | | |
| Candidaalbicans | 75 | 0.98-31.25 | 0.488-7.81 | 0.98-62.5 | | | 1.96-62.5 | 0.97-125 | 1.86-125 | | |
| Susceptibility of isolates from women in the control group |
| | Minimum inhibitory concentrations (MICs) | Minimum bactericidal concentrations (MBCs) |
| Organisms | No of strains | Cip | Amx | Ery | Tet | Kan | Cip | Amx | Ery | Tet | Kan |
| | μg/mL | μg/mL |
| Proteusmirabilis | 1 | 15.62 | 37.5 | 62.5 | 350 | 93.75 | 15.625 | 75 | 125 | 350 | 375 |
| Klebsiellaoxytoca | 1 | 1.96 | 2.25 | >250 | 350 | >375 | 1.98 | 4.49 | >250 | > 350 | >375 |
| | Minimum inhibitory concentrations (MICs) | Minimum Fungicidal Concentrations (MFCs) |
| | Clot | Ket | Fluc | | | Clot | Ket | Fluc | | |
| | μg/mL | μg/mL |
| Candidastellatoides | 3 | 0.98-7.81 | 0.98-3.90 | 15.63-62.5 | | | 1.96-31.25 | 1.96-15.63 | 62.5-125 | | |
| Candidatropicalis | 2 | 1.96-3.90 | 0.488-3.90 | 1.96-7.81 | | | 3.90-31.25 | 0.96-7.81 | 3.96-31.25 | | |
| Candidaalbicans | 3 | 0.98-31.25 | 0.98-7.81 | 0.98-31.25 | | | 1.96-31.25 | 0.97-125 | 1.86-125 | | |
Cip: Ciprofloxacin; Amx: Amoxicillin; Ery: Erythromycin; Tet: Tetracycline; Kan: Kanamycin; Cot: Clotrimazole; Ket: Ketoconazole; Flu: Fluconazole
Discussion
The increasing level of contraceptive use among women of child bearing age is an important component of many national population and developmental programmes in sub-Sahara Africa. In this study, women in age group 31-35 years were the largest group of contraceptive users and with the highest microbial colonisation. Among the 406 women volunteers studied, 162 (39.9%) showed microbial colonisation, with different species of microorganisms. While 106 (26.1%) women had different Candida species and 50 (12.3%) women were colonised with different bacterial strains, 1.5% of the study population had Trichomonas vaginalis. The steroid based contraceptive users had higher prevalence of candida colonisation (19.7%) when compared with barrier (4.4%) and control group (2.0%). The increase in colonisation of Candida species with barrier users is in agreement with earlier reports of Enweani IB et al., and Egbe CA et al., [13,20]. The isolation of these different microbial strains could be attributed to the exposure of the vaginal to different levels of gynaecological-obstetric risks [31]. Hyperestrogenemia resulting from certain mechanisms directly mediated by estrogens resulting in increase in vaginal glycogen, reduction of vaginal pH and easier adhesion to epithelial cell [32] has also been associated with the high microbial colonisation of the vagina.
Also, when compared to other contraceptive users, the bacterial colonisation was more associated with IUD users in the age group 31-35 years. This is in agreement with Yusuf A et al., that reported bacteria flora as being more prevalent among women in age group 26-35 years [33]. However, the presence of S. aureus, E. coli, Kleb. oxytoca and P. mirabilis may be attributed to lack or insufficiency of personal hygiene and transfer of faecal organisms from the anus into the vagina during cleaning. While this may be due to the shortness and wider nature of the female urethra and its proximity to the anus, alteration in vaginal microflora [34], movement of bacteria from the rectum up the urethra [35,36] and insertion of IUDs [37] have been suggested. While the isolation of T. vaginalis was very low (1.5%), more strains were isolated among women in ages 20-25 years using combined hormonal contraception than were obtained in other age groups. This is in agreement with Daran S et al., and Gavgani ASM et al., that reported higher rate of isolation in women less than 20-year-old and higher level of sexual activity in these women may account for the high rate of infection [38,39]. This finding suggested that colonisation of the protozoa may occur when women are young but disappear with age and, possibly, increase in sexual activity.
Although there is a dearth of information on the susceptibility of microorganisms isolated from asymptomatic women using contraceptives, the antimicrobial susceptibility testing showed that they were mostly resistant to antimicrobial agents used. The degree of resistance exhibited by these isolates may be attributed to the previous exposure of the women to different antibiotics at various times and the great potentials of the isolates to acquire resistance through varied mechanisms. The influence of the various contraceptives in promoting acquisition of resistance may not be underestimated. Thus, understanding the demographic and distribution of asymptomatic women using contraceptives may not be enough, detection and investigating the susceptibility profiles of microorganisms associated with them would give a better understanding of their clinical relevance. Early detection of these pathogens is important in preventing treatment failures while the knowledge of their sensitivity to antimicrobials can help to initiate empirical therapy against their potential infections [40].
Limitation(s)
The study was conducted in hospitals where treatment bills are paid by the patients. So, the study might have missed some of the women who could not attend the hospitals for same reason.
Conclusion(s)
In conclusion, this study showed that women in the age group 31-35 years were using combined hormonal contraceptives, Intra-Uterine Devices, latex condom and other contraceptive methods and are more at risk of reproductive tract infections. While the study indicated that the vaginal microbial isolates with pathogenic potentials in asymptomatic women using the different contraceptive methods, were highly resistant. The degree of these microbial resistances may be attributed to the unknown influence of the different contraceptives in promoting resistance acquisition by the microorganisms and previous exposure of the women to different antibiotics. While women desiring the use of contraceptive should be guided and fully educated on reproductive tract infections, signs and symptoms, if possible, sexual health education should also be extended to their partners in order to maintain healthy life.