One of the most common side effects of anticancer drugs is disruption of spermatogenesis process, leading to infertility in many cases [1]. BUS is a DNA-destruction chemotherapy agent used in low doses for long-term treatment of chronic myelogenous leukaemia and ovarian cancers, and in high doses for the treatment of bone marrow suppression in patients under bone marrow transplantation [2]. BUS inhibits cell division and has adverse effects on the cells with a high division rate, thereby exerting its highest impact on spermatogonial cells [3]. BUS also causes chromosomal sperm anomalies and lethal mutation mostly in sperms [4].
Boujrad N et al., reported that the consumption of BUS induces gonadal dysfunction in pregnant women and reduces somatic and germinal cells in the testis of infants [5]. Application of BUS as a proper pharmaceutical method to evacuate seminiferous tubules has long been taken into consideration in order to study the performance of germinal stem cells [6]. BUS can induce cell death through the production of free radicals [7], which impairs the lipids, proteins, and nucleic acids of cells [8]. The oxidative stress status due to the production of free radicals decreases the antioxidant enzyme activity, increases ROS level, and induces lipid oxidation consequently [9]. This phenomenon, in turn, causes the DNA breakdown and inactivation of specific proteins and consequential loss of biologic membranes [10].
Materials and Methods
An experimental study was conducted in which 64 male Wistar rats of 8 weeks and 220-250 gm were selected for the investigation. This study was done in Department of Anatomical Sciences, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran, from January 2019 to June 2019. It was conducted according to the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines following with approval of the experimental protocol from Institutional Animal Ethics Committee, Research Deputy of the University (Ethic number: IR.KUMS.REC.1398.0156).
The rats were maintained in an animal home with regular diet, water ad libitum, 12:12 hours light/dark cycle, 23±2°C and the humidity of 50±5%. All animals were adapted to the new environment in a week. Total of 64 rats were sorted into 8 groups; 1st as normal control group (received normal saline), 2nd as BUS group (received a single dose of 10 mg/kg BUS), 3rd to 5th as THYM groups (received doses of 4.5, 9, and 18 mg/kg of THYM, respectively), 6th to 8th as THYM+BUS groups (received a single dose of 10 mg/kg BUS+doses of 4.5, 9 and 18 mg/kg of THYM). All drug administrations applied by i.p. injection at 10 A.M. BUS administrated for induction of reproductive damage by a single dose but the THYM prescribed in three different doses for 5 consecutive weeks.
Chemicals and measuring kits: BUS and THYM were bought from Merck Co. (Germany) and also formalin, sodium acetate, ferric chloride, Iron sulfate, haematoxylin-eosin, and zinc sulfate powder were purchased from Sigma Co. (USA). Biochemical analysis kits were bought from Pars Azmun Co. (Iran). Other buffer additives and solvents were obtained from Merck Co. (Germany). 4.5 mg/kg of THYM prepared as follows; 0.54 mg of THYM was dissolved in normal saline (0.9%) to a volume of 1 cc, then it was passed through a 0.45-mm pore size filter (Lida Manufacturing, Kenosha, Wis. USA). Each treatment animal received 200 microlitres of THYM intraperitoneally. The other doses were made similarly and were injected immediately. For the formation of 10 mg/kg BUS (C6H14O6S2), 40.5 mg of the substance was dissolved in 6 mL of saline (1 mL for each rat by oral).
Dissection and sampling: The rats were anaesthetized by i.p. injection of ketamine HCl (100 mg/kg) and Xylazine (10 mg/kg). The heart blood sample was taken by the sub xiphoid aspiration method. Following preservation of the samples in a 37°C incubator for 20 minutes, they were centrifuged 255 g (1000 RPM, 15 minutes). The blood serum was isolated which was kept at -70°C for biochemical evaluation, including Total Antioxidant Capacity (TAC) and testosterone levels. In laparotomy procedure, the epididymis tail was separated and placed in DMEMF12/FBS5% culture medium. The left testis was removed and fixed in a 10% formalin solution for histological and morphometrical examinations and the right one was used for the MDA level estimations [12].
Sperm cell collection: Epididymides were cut in a petri dish (37°C). For sperm release, suspension of Hank’s balanced salt solution (10 mL) was prepared [17].
Progressive motility: Three degrees of sperm motility were considered based on WHO methods: 1.) Immotile sperms (Motility I); 2.) Non-progressive sperm (In situ sperm) (Motility II); 3.) progressive motility (Motility III). This value was examined with an optical microscope (40X) in 10 fields of view. For this purpose, 50 μL of the Semen liquid culture medium was placed on an alcohol sterilised slide culture. Then the slide culture was placed on it and examined with a microscope. Sperm counting was performed through a cell count device, and in each sample, 100 sperms were counted. To minimise the observer subjectivity, all sperm parameters, assessment was applied by two qualified expert technicians [10].
Sperm viability: Eosin staining was hired to identify the living sperm cells by absorption of stain by the dead cells and its disposal in living cells. At the end of the preferred time, 20 μL of the medium containing semen fluid was collected from each dish, and mixed with an equal volume of eosin stain solution (20 μL). Following 2-5 minutes, part of the mixture was transferred on a neobar slide culture. The living sperms lack stain, and dead sperms became pink. By magnification of 40X, at least 100 sperms were calculated from each random sample from the 10 fields of imagining and the percentage of live sperm cell was recorded [9].
Sperm cells morphology: Sperm smears were obtained from the right cauda epididymis followed by eosin/nigrosine staining. One drop of eosin stain was added to the suspension and mixed slightly; then 400 spermatozoa were observed by a light microscope (400X) for morphology abnormalities [17].
Sperm cells counting: To analyse the quantity of sperm cells, 400 μL of suspension was diluted with 10% formalin in Phosphate Buffered Saline (PBS) (Sigma; USA). A 15 μL of the previous solution was placed on a haemocytometer. It was then located in a Petri dish to be counted per mm3 [9].
Tissue staining for histological analysis of germinal layer in seminiferous tubules: The samples were placed in 10% formalin for 72 hours. Paraffin-embedded blocks prepared from each slab, were cut into 5 μm sections (LEICA SM2010RV1.2 Microtome Machine, Germany), and stained using hematoxylin and eosin (H&E) to analyse the height of germinal epithelium in testicular tissue. The slides were analysed according to previous study under a Olympus light microscope (Olympus microscope ECLIPSE E600W, Tokyo, Japan) equipped with DP12 cam (KEcam Technologies, Lekki Lagos, Nigeria) with stereological probes and using Adobe Photoshop CC (Olympus Optical, LTD, Tokyo, Japan) [18].
Testosterone level: Blood samples were aspirated from the heart, and serum was collected by centrifugation (3000 RPM, 15 minutes). The serum level of testosterone was measured by ELISA method (Abcam 108666, USA) [10].
Measurement of testis MDA: In right testis, the MDA levels were evaluated as an index of lipid peroxidation. In this regard, the homogenised samples were obtained by homogenization buffer 1500 g (12,000 RPM, 10 minutes). The samples were added to a reaction mixture containing SDS, acetic acid (pH 3.5), thiobarbituric acid, and distilled water. The mixture was boiled and centrifuged at 3000 g, (10000 RPM, 10 minutes). Finally, the supernatant absorbency was measured (550 nm) [18].
Estimation of testis total antioxidant capacity: An acquisition kit (Cat No: TAC-96A, ZellBio GmbH-Germany) was purchased to measure the TAC level based on the oxidation colourimetry resuscitation; thus the TAC was equivalent to antioxidant level in the sample in comparison with ascorbic acid as standard value. The kit contains buffer X 100, dye powder, reaction suspension solution, standard and a microplate of 96 wells. The sensitivity of the kit was equal to 0.1 mM, and final absorbance was read at 490 nm, and unit conversion was performed [18].
Statistical Analysis
The data were analysed by SPSS software (version 20.0) using one-way ANOVA postulation followed by Tukey post-hoc test. p<0.05 was considered as significant, and the variables were represented as the mean±standard error of mean.
Results
Progressive sperm motility and viability: BUS administration caused a significant reduction in sperm viability and progressive motility compared to the normal control group (p<0.01). No significant variations were detected in THYM groups in comparison with the normal control group (p>0.05). Also, sperm cell viability and progressive motility in all treated THYM and BUS+THYM groups increased significantly compared to the BUS control group (p<0.01, [Table/Fig-1]).
Effect of BUS and THYM on sperm parameters in male rats (n=8 for each group).
| Groups | Sperm count (106) | Progressive motility (%) | Sperm viability (%) | Normal morphology (%) |
|---|
| Normal control | 85.37±1.06 | 19.6±1.32 | 75.53±1.16 | 81.37±2.43 |
| BUS control | 31.16±4.05* | 1.87±1.40* | 40.83±3.05* | 36.53±3.16* |
| THYM 4.5 mg/kg | 85.75±2.43† | 21.12±1.21† | 76.62±2.09† | 81.75±4.51† |
| THYM 9 mg/kg | 86.12±5.07† | 20.87±1.74† | 76.55±5.04† | 81.37±4.66† |
| THYM 18 mg/kg | 85.25±4.07† | 20.50±0.67† | 75.05±1.07† | 82.50±2.74† |
| THYM+BUS 4.5 mg/kg | 49.50±2.50¶ | 7.12±1.33¶ | 54.35±5.08¶ | 55.13±1.46¶ |
| THYM+BUS 9 mg/kg | 51.36±3.17¶ | 8.87±1.51¶ | 56.37±2.09¶ | 57.14±4.22¶ |
| THYM+BUS 18 mg/kg | 55.25±4.23¶ | 8.75±1.10¶ | 57.21±3.51¶ | 59.87±1.58¶ |
Data are presented as mean±SEM and analysis using one-way ANOVA. *p<0.01 compared to the normal control group. †p<0.01 compared to BUS control group. ¶p<0.01 compared to the BUS control group. THYM: Thymoquinone, Busulfan; BUS: Busulfan
Sperm count and normal morphology: BUS caused a significant reduction in sperm count and morphology in the BUS control group compared to the normal control group (healthy) (p<0.01). At all doses of THYM extract treatment groups, the sperm count and morphology were increased significantly compared to the BUS control group (p<0.01) [Table/Fig-1].
Height of germinal layer in seminiferous tubules: BUS led to a significant reduction in the height of the germinal layer in comparison with the normal control group (p<0.01). No significant alterations were witnessed in comparison with the normal control group (p>0.05). The height of germinal layer in entire groups of THYM and BUS+THYM was improved significantly compared to the BUS control group (p<0.01) [Table/Fig-2,3].
Effect of BUS and THYM on height of germinal layer in seminiferous tubules and testosterone hormon level in male rats (n=8 for each group).
| Groups | Germinal layer height (μm) | Testosterone (ng/mL) |
|---|
| Normal control | 51.87±0.9 | 3.88±0.1 |
| BUS control | 24.5±3.05* | 1.04±0.20* |
| THYM 4.5 mg/kg | 52.62±2.43† | 4.12±0.34† |
| THYM 9 mg/kg | 53.27±2.07† | 4.57±0.14† |
| THYM 18 mg/kg | 51.25±3.33† | 4.37±0.3† |
| THYM+BUS 4.5 mg/kg | 36.87±1.50¶ | 1.98±0.44¶ |
| THYM+BUS 9 mg/kg | 37.87±3.67¶ | 2±0.11¶ |
| THYM+BUS 18 mg/kg | 37.35±2.13¶ | 2.32±0.31¶ |
Data are presented as mean±SEM and analysis using one-way ANOVA. *p<0.01 compared to the normal control group. †p<0.01 compared to BUS control group. ¶p<0.01 compared to the BUS control group. THYM: Thymoquinone, BUS: Busulfan
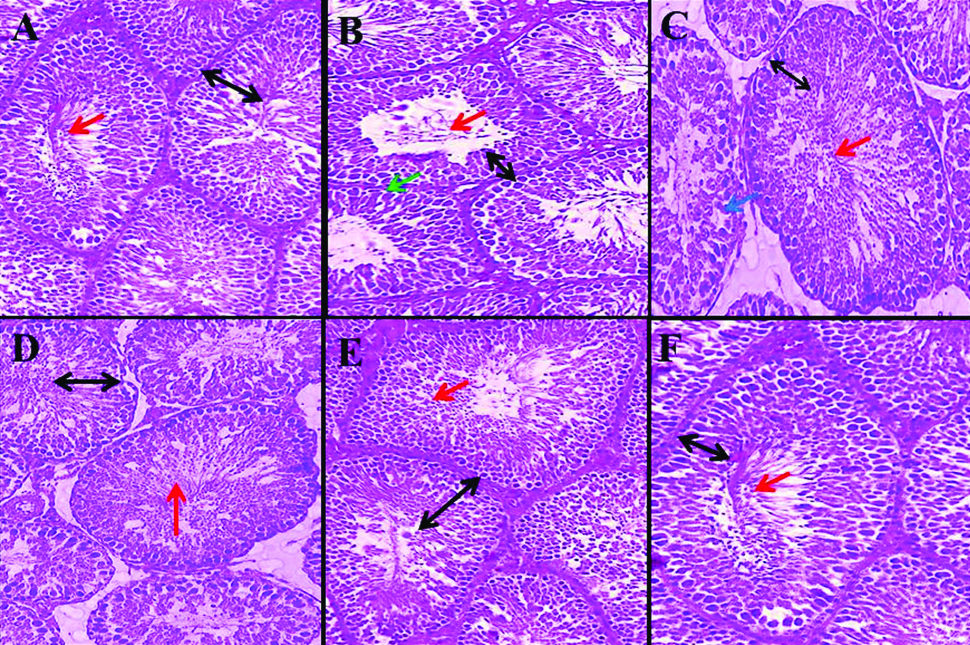
Normal seminiferous tubule structure was observed in normal control (A), THYM (18 mg/kg) (F), THYM+BUS (9 mg/kg) (D) and THYM+BUS (18 mg/kg) (E). A decrease in height of the germinal layer, destruction of the cells sequence, vacuolization and reduce sperm cells density were observed in BUS control (B and C). Black identifies germinal layer height, red identify sperm cells density, green identifies irregularities in the margin of tubules (destruction of the membrane seminiferous tubules structure), and blue identifies vacuolization. THYM: Thymoquinone, BUS: Busulfan. (Eosin Stain, 40X)
Testosterone: BUS caused a significant decrease in the testosterone hormone level compared to the normal control group (p<0.01). No significant alterations were detected in THYM groups in comparison with the normal control group (p>0.05). Furthermore, the level of testosterone in the whole treated THYM and BUS+THYM groups improved significantly compared to the BUS control group (p<0.01) [Table/Fig-2].
MDA levels: Serum levels of MDA showed significant growth in the BUS control group compared to the normal control group (p<0.01). Also, a significant reduction in MDA levels was shown in all THYM and THYM+BUS groups compared to the BUS control group (p<0.01) [Table/Fig-4].
Effect of BUS and THYM on MDA and TAC levels in male rats (n=8 for each group).
| Groups | MDA (nm/gKW) | TAC (mmol/mL) |
|---|
| Normal control | 70.94±0.62 | 1.08±0.02 |
| BUS control | 117.13±2.64* | 0.41±0.04* |
| THYM 4.5 mg/kg | 67.63±4.03† | 1.11±0.07† |
| THYM 9 mg/kg | 67.19±5.05† | 1.12±0.03† |
| THYM 18 mg/kg | 67.40±0.93† | 1.11±0.03† |
| THYM+BUS 4.5 mg/kg | 98.93±4.70¶ | 0.88±0.08¶ |
| THYM+BUS 9 mg/kg | 96.98±2.47¶ | 0.85±0.04¶ |
| THYM+BUS 18 mg/kg | 96.38±5.23¶ | 0.89±0.01¶ |
Data are presented as mean±SEM and analysis using one-way ANOVA. *p<0.01 compared to the normal control group. †p<0.01 compared to BUS control group. ¶p<0.01 compared to the BUS control group. THYM: Thymoquinone, BUS: Busulfan, MDA: Malondialdehyde, TAC: Total antioxidant capacity.
TAC levels: The results of measured TAC levels in the study groups showed a significant decrease in the BUS control group compared to the normal control group (p<0.01). Also, a significant increase in TAC levels was found in all THYM and THYM+BUS groups compared to the BUS control group (p<0.01) while no significant effect on the levels of TAC in all THYM groups compared to the normal control group was found (p>0.05) [Table/Fig-4].
Discussion
The findings of the current research suggested that BUS administration had adverse and destructive effects on testis histology and sperm parameters, oxidant-antioxidant imbalance as well, and increase in testosterone hormone level. THYM alleviated the destructive effects of BUS on male reproduction system. This extract also recovers testicular damages by reduction in levels of MDA and lipid peroxidation and also increment in cellular anti-oxidant capacity as it was stated in various studies [11-13]. The results of the present study showed that all sperm parameters in the BUS control group reduced significantly compared to the normal control group. In THYM and BUS+THYM groups, a significant increase was observed in all sperm parameters compared to the BUS control group. The findings of Aitken RJ and Baker MA, confirmed the results of the present study in that oxidative stress disturbed spermatogenesis and caused defective gametes with remodelled chromatin, which were vulnerable to the attack by free radicals and reduced the number of spermatogonia, spermatocytes, and spermatozoa [19]. Reduction of all sperm parameters in BUS group might be due to the direct increase of oxidative stress-induced lipid peroxidation, which could have been able to alter the natural properties of the membrane and therefore cause the loss of sperms transferring to epididymis as well as those within epididymis [20].
On the other hand, high levels of ROS induces mitochondrial damage and consequently causes the release of pro-apoptotic proteins within the intermembrane space, activation of caspases, reduction of ATP synthesis process, release more amount of ROS, increase of intracellular calcium concentration and calcium transfer from mitochondria to cytosol, which in turn may lead to activation of apoptosis process [9].
BUS can induce DNA damage and destruction cell by increasing the expression of STRA8, MAK mRNA, and RAD51 [21]. Bahmanpour S et al., reported the administration of BUS for male Wistar rats which attenuated the number of spermatogonia, spermatids, Leydig and Sertoli cells compared to control group, confirming the results of the current research [21]. Salahshoor MR et al., showed that THYM administration improved sperm parameters, including count, motility, and viability in morphine treated rats, which is in line with the results of the present study [10].
The present study indicated that serum testosterone level and germinal layer thickness of seminiferous tubules was significantly attenuated in the BUS group than normal control group. There was also a significant increase in level of testosterone hormone and germinal layer height of seminiferous tubules in all THYM and THYM+BUS groups than BUS control group. Further, histological analyses showed loss of natural form, order, and consistency of cells on the walls of seminiferous tubules, creating vacuoles in them after all. Development of vacuole in the testis tissue can be indicative of the effect of the oxidative stress process. It seems that BUS as an alkylating agent causes cell destruction and DNA damage, thereby decreasing the thickness of the germinal layer in the testis [22]. The results of Olejnik J et al., were in agreement with the findings of the present research, indicating that BUS administration reduced the diameter of seminiferous tubules and sperm count, motility and viability, increased the abnormal sperms and decreased the epithelial thickness of seminiferous tubules [23].
As an antioxidant, THYM not only inhibits lipid peroxidation and testicular oxidative stress but also plays a key role in the production of steroids in testis [10]. In addition, THYM can reduce the effects of the oxidative stress-induced under various conditions and strengthen the cells to cope with these conditions by preventing the reduction of glutathione and increasing antioxidant capacity [24]. The findings of Waly H et al., were in line with those of the present study, showing that phytoestrogens, including THYM is attached to the estrogen receptors in testis and stimulates spermatogenesis through strategies such as increasing the epithelial layer, the diameter of seminiferous tubules, and lumen diameter [25]. The present study showed that BUS-induced male reproductive damage in rats could be reduced by plant antioxidants such as THYM.
Limitation(s)
The present study has certain restrictions; authors did not study in detail about the mechanism of THYM on the male reproductive parameters. There was a lack of references for the THYM. There were deaths of some animals due to BUS administration of this study and thus were excluded from the study. Hence prospective studies should be taken for detailed association of the molecular interaction between THYM, BUS and reproductive parameters.
Conclusion(s)
The outcomes of this study demonstrated that BUS produces defects in some of the male reproductive parameters and THYM has an antioxidant and defensive effect. It was revealed that THYM elevates the quality of spermatozoa and improves the typical morphology, sperm viability, germinal layer seminiferous tubules height, TAC, motility, and count and reduces testis MDA level. THYM can be valuable for the treatment of infertile men to enhancement male fertility. The antioxidant properties of THYM could be the main reason for its optimistic outcome on reproductive parameters. Supplementary studies are essential to explain their careful mechanism of action. However, it is suggested that THYM may have beneficial effect on reproductive parameters in patients with BUS administration.
Data are presented as mean±SEM and analysis using one-way ANOVA. *p<0.01 compared to the normal control group. †p<0.01 compared to BUS control group. ¶p<0.01 compared to the BUS control group. THYM: Thymoquinone, Busulfan; BUS: Busulfan
Data are presented as mean±SEM and analysis using one-way ANOVA. *p<0.01 compared to the normal control group. †p<0.01 compared to BUS control group. ¶p<0.01 compared to the BUS control group. THYM: Thymoquinone, BUS: Busulfan
Data are presented as mean±SEM and analysis using one-way ANOVA. *p<0.01 compared to the normal control group. †p<0.01 compared to BUS control group. ¶p<0.01 compared to the BUS control group. THYM: Thymoquinone, BUS: Busulfan, MDA: Malondialdehyde, TAC: Total antioxidant capacity.