Traumatic Brain Injury (TBI) results from a violent external mechanical force to the head or body, such as a motor vehicle crash, a sports injury, or a simple fall to the ground, that causes brain dysfunction [1]. Traumatic brain injury is a cause of high mortality and morbidity and is an area of intense research and also the least researched area. Apoptosis plays a crucial role in the pathogenesis of head injury, and the inhibition of apoptosis can potentially reverse the deleterious effects and lead to a better functional outcome [1].
Tumour suppressor gene, p53 is known to be pro-apoptotic. The p53 acts through several pathways as a response to cellular stress. Either it can arrest cell cycle at the G1 checkpoint or bring about apoptosis and cellular senescence [2]. This ensures either DNA repair or programmed cell death, minimising the chances of mutations and cancer. Damage to p53 gene results in the conversion of proto-oncogenes to oncogenes. This fact is supported by the observation that transgenic mice over expressing p53 were virtually immune to the development of tumours [3].
Even though increased p53 activity is beneficial for cancer resistance, mice over expressing p53 aged prematurely and died earlier than their wild-type counterparts [3]. The beneficial effect of activated p53 is observed in young organisms. But it might reduce lifespan and even enhance cancer risk, called antagonistic pleiotropy in older organisms [2,4,5]. Campisi J, supported these findings by stating that p53 accelerates aging when responding to cellular stress [6]. Thus expression of the gene for p53 may be a double-edged sword.
The p53 has different genotypes expressions, homozygotic Arg72Arg, heterozygous Arg72Pro, and homozygous Pro72Pro. These genotypes variants were discovered due to the difference in their electrophoretic mobility [7]. The SNP of p53 has been implicated in outcome for patients with TBI [8]. There are marked functional differences between the two forms, Arg/Arg and Arg/Pro of the p53-protein [9-12]. The Arg72Arg form is more efficient in apoptosis induction, whereas the Arg72Pro form arrests cell cycle at G1 and its main role is to activate p53 dependent DNA repair [13-16]. The frequency of the Pro72Pro allele of Arg72Pro ranges from 70% among South Africans to 23% among Western Europeans. The gradient from Europe to Africa according to latitude suggests that the Pro72Pro allele is a better protector against sunlight-induced diseases [17], however, the few epidemiological studies examining the Arg72Pro genotype and risk of skin cancer disagree with this [18,19].
The reduced mortality in Arg/Pro heterozygotes and Pro/Pro homozygotes versus Arg/Arg homozygotes, which could result from a generally increased robustness caused by decreased pro-apoptotic activity and increased cell cycling arresting abilities of the Pro72 versus the Arg72 version of p53, thereby protecting a person experiencing any critical illness [10,14,20].
Recovery after TBI is heterogeneous and depends on the age of the patient, and the nature, location, and extent of the injury [21,22]. The known predictors account for only a limited percentage of the variation in outcomes. Recent studies indicated that genetic variants, such as the p53 polymorphism, may also contribute to the severity and outcome of TBI. However, only a few studies which study the association of p53 gene polymorphism and functional outcome after TBI are available to the best of our knowledge. There is no meta-analysis done on this aspect. We have undertaken this meta-analysis so that results can be conclusive with a higher statistical power.
Materials and Methods
The present meta-analysis was carried out in February 2019 to August 2019 at Department of Biochemistry, KS Hegde Medical Academy, Nitte (Deemed to be University), Mangaluru, Karnataka, India.
Publication Search
The research articles published between 2000-2019 were selected from Google Scholar, PubMed, and Scopus databases by using the search keywords: “p53 gene polymorphism AND traumatic brain injury”. The published original research articles which are written in English language and matching the eligibility criteria were retrieved. Only the full text original articles with required information were included in the meta-analysis.
Inclusion Criteria
Case control studies on Arg72Pro polymorphism of p53 gene in TBI with sufficient genotype distribution data, containing information about available genotype frequency that can help infer the results in the studies, published studies with full-text articles as well as abstracts with essential information were included in the analysis.
Exclusion Criteria
Duplicates or overlapping population studies, studies with incomplete information were excluded from the analysis.
Data extraction
As per the eligibility criteria, we could get only four studies. Data were extracted from the three eligible publications [8,23,24]. From each study, the following characteristics were collected: the first author’s last name, publication year, country of origin, ethnicity, genotyping methods and outcome after TBI. Different ethnic descents were categorised as Caucasian and Asian. Ethical approval was not required as it was a systematic review of published articles.
The p53 genotype data for patients with different GOS or GOSE scores, or for patients with favorable and unfavorable functional outcomes, or odds ratio and the corresponding 95% CI were collected. For studies that reported p53 data for different GOS or GOSE scores, we dichotomised the GOS or the GOSE score into favourable and unfavourable outcomes, with GOS 4 or GOS 5 being a favourable or good outcome, GOS score less than or equal to 3 including death were considered to be a poor outcome.
The association of p53 gene polymorphism with the TBI patients’ functional outcome at discharge was assessed. It would be better if studies were available which assess functional outcomes at different intervals like six months, one year and so on.
Statistical Analysis
The statistical analysis was carried out using the software Medcalc. The frequency of homozygous alleles Arg/Arg versus Arg/Pro heterozygous and Pro/Pro homozygous alleles were estimated and their association with the functional outcome was assessed.
The association of functional outcome (good or bad outcome) with different genotypes of p53, Arg/Arg, Arg/Pro, and Pro/Pro was assessed by pooled Odd’s ratio (OR) with 95% Confidence Interval (CI). Random effect models were used to calculate OR and CI. The significance of summary ORs was determined with a Z test. Heterogeneity assumption was checked by χ2-based Q test. A p-value more than 0.10 for the Q test indicated lack of heterogeneity among the studies, and the summary OR estimate of each study was calculated by the random-effects model (Der Simonian and Laird method) as well as by fixed-effect model (Mantel-Haenszel method) [25]. In the absence of individual heterogeneity, all the points were expected to lie within the confidence bounds [26,27].
Between-study heterogeneity was assessed using I2. Publication bias was evaluated using a funnel plot and the standard error of log (OR). Statistical significance was defined as p<0.05.
Results
Study Selection and Characteristics
The selection process for the eligible studies included in the meta-analysis is shown in [Table/Fig-1]. Original research articles as per the eligibility criteria included were only four. One article was excluded as the complete citation was not traceable. Out of three studies included in the meta-analysis, one study had evaluated the functional outcome after TBI at discharge and at six months. But the other two studies had assessed the outcome at discharge. The meta-analysis evaluated the association of p53 gene polymorphism with outcome after TBI at discharge.
Flow chart showing the selection of articles of meta-analysis.
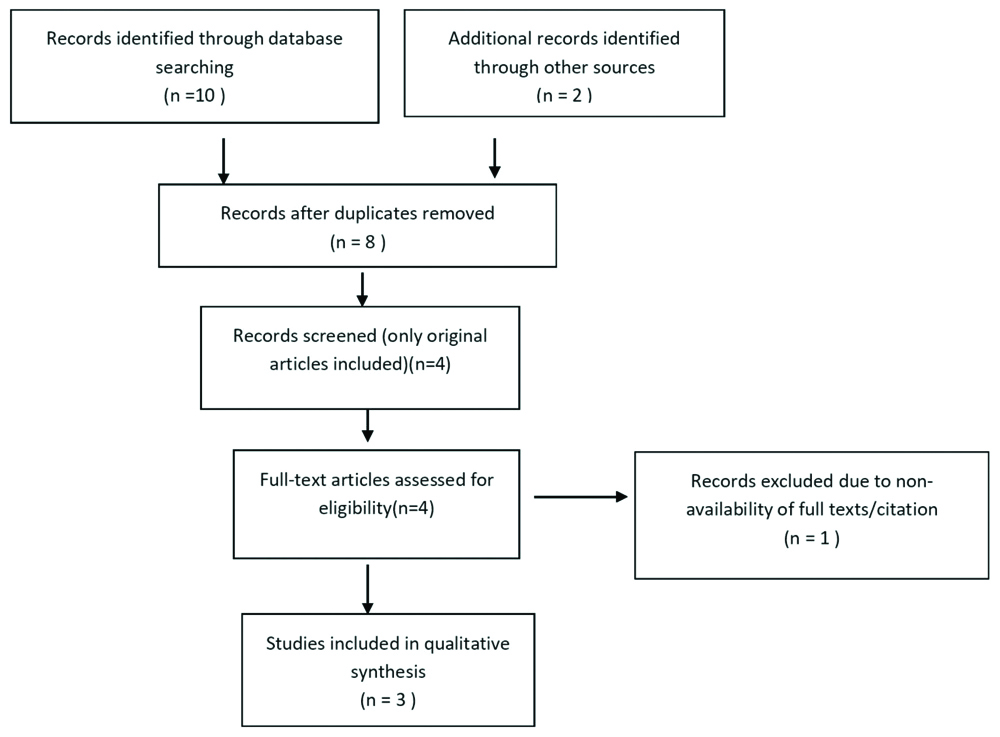
As the study includes three original articles, a total sample size was 231 TBI cases, out of which 127 patients had a poor prognosis at discharge. The poor outcome is defined in terms of Glasgow Coma Scale (GCS), GCS of 1 representing death, 2 being vegetative state and 3 being unable to live independently but able to follow commands. Hundred and four patients showed a good outcome, which was defined as Glasgow coma scale 4 and 5, which represent ability to live independently but unable to return to work and ability to return to work respectively. Ninety nine patients with a poor outcome showed Arg/Arg genotype and 28 of them showed Arg/Pro plus Pro/Pro genotypes. Sixty four patients with good outcome showed Arg/Arg genotype and 40 patients with Arg/Pro plus Pro/Pro genotype, showed a better outcome [Table/Fig-2].
Characteristics of selected studies.
| References | Year of publication | Ethnicity | Sample size | Polymorphism in poor outcome (Arg vs Pro) | Outcome assessment | Follow-up |
|---|
| Martinez PL et al., [8] | 2005 | Caucasians | 90 | 42/19 | GOS | Discharge, six months |
| Martinez PL et al., [23] | 2009 | Caucasians | 90 | 52/07 | GOS | Discharge |
| Mohammed Ali HA et al., [24] | 2016 | Africans | 51 | 05/02 | GOS | Discharge |
Studies may have heterogeneity due to patients, outcome definition and study design. Both random effect model as well as the fixed effect models were used to assess the association between the gene polymorphism and outcome. Fixed effect model assumed that the effect of polymorphism is the same on both good and bad outcomes.
Forest plot by random effect model [Table/Fig-3] for the association of bad outcome and Arg/Arg polymorphism of the p53 gene showed that only one study was passing the line of no effect. Rest of two studies were on the right side of the line of no effect which suggested that A/A polymorphism of p53 was favouring bad outcome. As the size of individual squares were directly proportional to the weight of the study, both the studies of significance were given the highest weight.
Forest plot for analysing the association between p53 gene polymorphism and outcome after TBI.
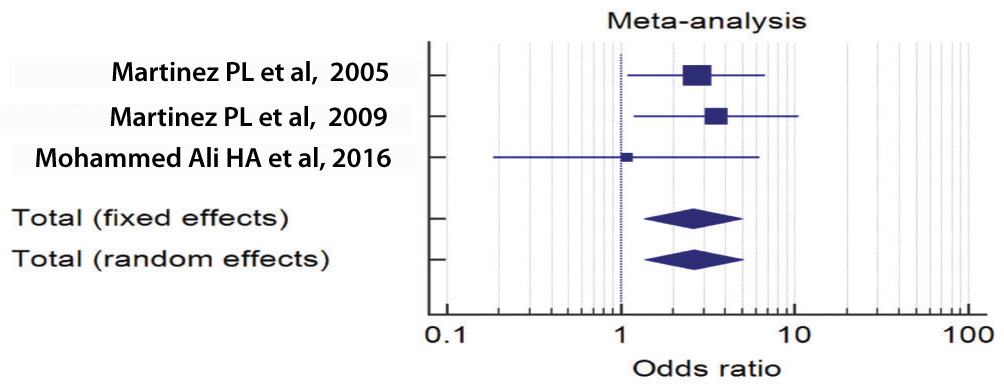
Diamonds of both fixed and random effect models were on the Rt side of the line of no effect, which clearly suggested that Arg/Arg polymorphism of p53 was associated with poor outcome. Z values for random and fixed effect models were 2.923 and 2.875, CI values being 1.376-5.048 and 1.357-5.012 respectively, p=0.003 and p=0.004 respectively for both the models.
Q test for heterogeneity was 1.261, the degree of freedom, DF=2 and p=0.532 suggesting that there was not much heterogeneity between studies. There was no inconsistency, I2=0% 95% CI=0.00-94.68. ORs were 2.636 and 2.608 respectively for random and fixed effect models [Table/Fig-4].
Erpretations of results of the forest plot.
| Variables for studies | Study |
|---|
| Intervention groups |
| Variables for total number of cases | Total bad prognosis |
| Variables for total number of positive cases | P53 Plymorphism A A Bad outcome |
| Control groups |
| Variables for total number of cases | Total good prognosis |
| Variables for total number of positive cases | P53 Plymorphism A A Bad outcome |
| Study | Intervention | Controls | Odds ratio | 95% Cl | z | p-value | Weight (%) |
| Fixed | Random |
| Martinez PL et al., [8] | 42/16 | 13/29 | 2.721 | 1.094-6.763 | | | 50.93 | 50.93 |
| Martínez PL et al., [23] | 52/73 | 7/17 | 3.537 | 1.188-10.529 | | | 35.50 | 35.50 |
| Mohammed Ali HA et al., [24] | 5/7 | 30/43 | 1.083 | 0.186-6.324 | | | 13.57 | 13.57 |
| Total (fixed effects) | 99/141 | 50/89 | 2.608 | 1.357-5.012 | 2.876 | 0.004* | 100.00 | 100.00 |
| Total (random effects) | 99/141 | 50/89 | 2.636 | 1.376-5.048 | 2.923 | 0.333* | 100.00 | 100.00 |
| Test for heterogeneity |
| Q | 1.2609 |
| DF | 2 |
| Significant level | p=0.5324 |
| I2 (inconsistency) | 0.00% |
| 95% CI for I2 | 0.00 to 94.68 |
*Significant; Q value: Heterogeneity coefficient; DF: Degree of freedom
Funnel plot for publication bias was symmetrical, inverted funnel-shaped, boundaries being straight lines. The studies are symmetrically distributed in the plot suggesting that no publication bias is present [Table/Fig-5].
Funnel Plot to analyse publication bias: X-axis is the OR and Y-axis is standard error.
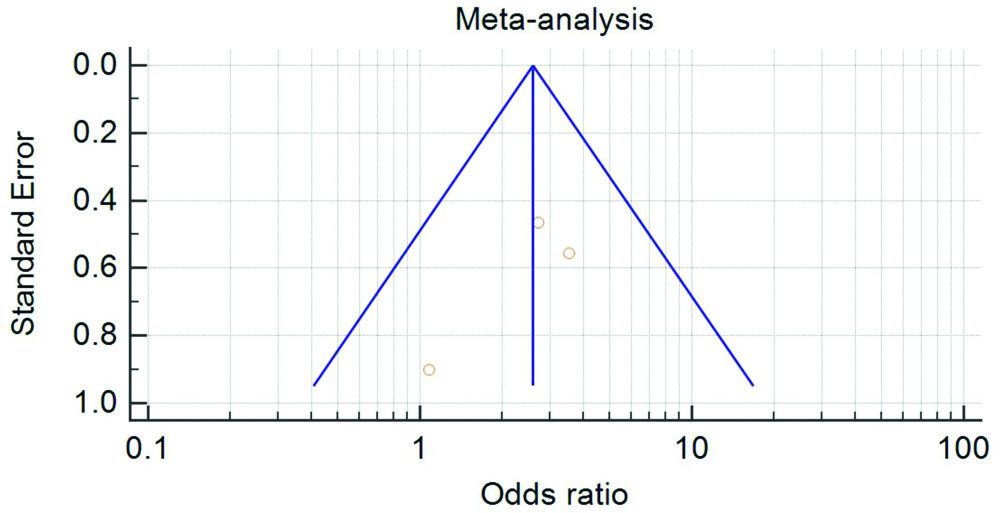
Each study is represented by a square, area of which is directly proportional to the weight of the study. Diamond represents overall weight of the study, width of which represents 95% CI for the estimated OR.
Discussion
There are no meta-analysis or systematic reviews available on this topic to the best of our knowledge. The study seems to be the first meta-analysis on the association of Arg72Pro polymorphism of p53 on the functional outcome after TBI. The meta-analysis suggests that Arg/Arg polymorph of p53 is associated with poor outcome at the discharge of TBI patients.
The study by Martinez PL et al., published in 2005 was a prospective study carried out on 90 Caucasian patients with severe TBI and 100 controls. The study reported no significant differences between frequencies of Arg72Pro polymorphisms compared to controls. Outcome measurement tool for TBI was Glasgow outcome scale at discharge and six months. The results suggested that 69% of bad outcomes were associated with Arg/Arg genotype. It also revealed that Arg/Arg variants had a 2.9 times greater risk of having a bad outcome at discharge. There was no significant difference in the length of stay at the hospital between the patients with Arg/Arg genotype and Arg/Pro and Pro/Pro genotypes. The study did not report any association between mortality and Arg/Arg polymorphism [8].
There was a statistically significant difference in the genotypes and bad outcome at six months after TBI.
The association between genotypes and outcome at six months could not be included in the meta-analysis because the other two reports, Martinez PL et al., and Mohammed Ali HA et al., studied Arg72Pro polymorphs and Glasgow outcome scale at only discharge [23,24].
Martinez PL et al., recruited 90 patients with severe TBI patients and Glasgow outcome scale at discharge being the outcome assessment tool, reported that 81.1% of patients had a poor outcome and 18.9% of them had a good outcome. The results clearly indicated that Arg/Arg polymorphism was an independent predictor of poor outcome, the risk of a poor outcome being 3.55 times greater with Arg/Arg genotypes which was in agreement with their previous report [23].
The study by Mohammed Ali HA et al., recruited 51 Sudanese TBI patients, out of which 84.31% were discharged with a good outcome. 69.76% of patients with good outcome had Arg/Arg genotype, 11.62% had Arg/Pro and 6.98% had Pro/Pro genotypes. Around 13.72% of patients showed a poor outcome, 71.42% of them died and 28.57% had a poor prognosis. Probably one patient could not be followed-up. The study concludes that 100% of patients with Arg/Pro and Pro/Pro alleles survived [24].
Limitation
Due to paucity of published studies in this particular field, this study could include only three original articles. Outcome assessment of TBI patients was done only at discharge. There is a need to study the functional outcome periodically at discharge, six months, one year and association of p53 gene polymorphism with both short as well as long-term outcomes have to be established.
Conclusion
It could be concluded from this meta-analysis that there is an association between Arg72Pro polymorphism of the p53 gene with the functional outcome after TBI at discharge. Patients with Arg/Arg homozygous genotype were found to be associated with poor outcome.