Urinary Tract Infection (UTI) is described as the presence of organisms in the urinary tract which is usually sterile [1]. The presence of significant number of bacteria in the urine of asymptomatic individuals denotes asymptomatic bacteriuria [2,3]; which will not alert the person to seek timely treatment and thus may present to hospital with complications like pyelonephritis and renal damage [3]. Lower UTI termed cystitis usually results from bacterial infection [1,4,5] and over 90% of cases are caused by Escherichia coli, other include Enterobacteriaceae, Staphylococcus saprophyticus, or Enterococcus [6-8]. The cardinal symptom of lower UTI is dysuria; other symptoms include dereased urinary frequency, nocturia, urgency, voiding small volumes, incontinence, and suprapubic or pelvic pain [4,5].
As per the World Health Organisation definition (2013), a child is a person 19 years or younger and when a person falls into the 10 to 19 age category they are referred to as an adolescent. The higher prevalence of UTI among adolescent girls is attributed to hormonal change causing alterations in vaginal microflora and migration of the nephritogenic bacteria to the periurethral region [13,16,17]. As reported by the National Family Health Survey 2000, the prevalence of UTI among adolescent girls in India is 16.6% [17]. Adolescent girls often have apprehension at menarche coupled with hesitancy to seek medical attention for symptoms of UTI [12]. It is observed that prevalence of bacteriuria in school girls rises steadily with age and repeated screening of school girls for UTI is a preventive measure [18].
As compared to the gold standard procedures of urine microscopy and urine culture, reagent strip nitrite and Leukocyte Esterase (LE) provide cost-effective and faster indirect confirmation of bacteriuria and leukocyturia, respectively [2]. Although reliability of urine dipstick manual assay in predicting UTI have been evaluated [14,19-28], there is paucity of epidemiological data on the feasibility of self screening for UTI. Two studies in England [19,29] and one study in Thailand [28] have analysed self performing and/or self interpreting the dipstick test for UTI. To our knowledge, the self screening approach adopted in the present study to predict UTI and negative urine cultures in selected population is new in India. In the present study the urban and rural school adolescent female population was evaluated for ability to self screen for UTI by dipstick method. An attempt was made to establish an association between UTI and various contributory factors like water intake, frequency of urinary voiding and perineal cleaning practices. The prevalence of UTI among adolescent girls in urban and rural schools was determined.
Materials and Methods
This was a cross-sectional study conducted over a three months period from June-August 2016. The study protocol was approved by the Institutional Ethics Committee (EC Reg No: ECR/747/Inst/KA/2015). The permission from Heads of Institutions of one rural school and two urban schools in the state of Tamil Nadu, India was obtained to seek participation of adolescent girls. The rural school included Ethiraj Matriculation School, Sathuvachari Village, Vellore District, comprising 400 female students. The urban schools included Baby Matriculation School and Ramana Vidyalaya School, Chennai together comprising 300 female students. A total of 377 participants including 186 girls from the rural school in Vellore and 191 girls together from the two urban schools at Chennai who consented were enrolled in the study.
The sample size was determined using the following formula:
N=Z2P(1-P)/d2.Using the prevalence of UTI, P=2.8% (≅3%) as reported by Asscher AW et al. [29], and with an absolute precision of 2%, the sample size was calculated to be a minimum of 280.
The inclusion criterion was girls in the adolescent age group (10-19 years). The exclusion criteria were all male students, any neurological abnormality or congenital anomaly of urinary tract, those who had taken antibiotics within the preceding 72 hours, girls going through their menstrual cycle and not interested to participate in the study. At each school an interactive session was conducted by the investigators to educate the girls about UTI and the objectives and methodology of the study. The procedure to perform and interpret the Multistix 10 SG Reagent Strip (Siemens Healthineers India) test as per the instructions of the manufacturer was demonstrated to the participants and ensured that they have understood it. A questionnaire [1,12,13,15,30-33] was provided pertaining to details of age, willingness to participate in the study, any urinary tract abnormality or persisting medical condition, receiving antibiotic therapy, symptoms of UTI, amount of Daily Water Intake (DWI), habit of holding urine, and method of perineal cleaning. Approval for participation in the study was obtained from the parents/legal guardian through a written informed consent form stating brief methodology, objectives and importance of the study, confidentiality of the identity and test results, non-compulsory participation and option to contact the investigator over phone or at school for any queries.
The confirmed participants at each school were divided into batches of 45-50 students per day and the test was performed on successive days. The participants were given verbal instructions for collecting the first-morning, clean-voided, midstream urine sample and were provided with a sterile labelled container. The participants were asked to report to the school (to the investigators) the following day with their urine sample within one hour of sample collection. Each day the batch was divided into subgroups and were provided with stop watch, colour chart and reagent strip. Every participant was asked to examine her sample for abnormal colour or appearance and test immediately for glucose, protein, nitrite and LE with dipstick method. The test results for glucose, protein, nitrite and leukocytes were read visually at 30 seconds, 60 seconds and two minutes, respectively in good light. Any degree of uniform pink colour was considered as positive for nitrite test. A LE strip result of small (+), moderate (++) and large (+++) as per the colour chart on the reagent strip container was considered as positive. The participants recorded their observation as positive or negative for the individual tests. At the same time, sample of every participant was analysed by the investigators as well and the test results were recorded. For the purpose of this study, UTI was considered to be present if the tests for nitrite and/or leukocytes were positive as per the investigator’s observation. If the sample was positive for glucose and/or protein (in accordance to the investigator’s observation), the participant was excluded from statistical analysis of data. The participants with dipstick positive result for protein, sugar and UTI were referred for further evaluation and urine culture analysis.
The bacteria-specific nitrite test depends on the conversion of nitrate to nitrite by bacterial action (>105-106 bacteria/mL) in the urine [2,34]. The nitrite test area of multistix is impregnated with p-arsanilic acid which reacts with nitrite to form diazonium salt which is followed by azo-coupling reaction to give a pink azo dye [2,20]. The host response-specific LE test [21] indicates presence of remnants of neutrophils in the urine that are not visible microscopically [27]. The leukocyte esterase hydrolyses the ester on the test area of multistix to form pyrrole alcohol which further reacts with diazonium salt to give purple colour [2,20]. Elevated urine protein and/glucose may decrease the result of LE test [2].
Statistical Analysis
Of the 377 participants, 374 were considered for statistical evaluation. Three participants who tested positive for protein were not considered. Statistical analysis was performed using SPSS version 21.
The ability to self test was assessed statistically by evaluating sensitivity, specificity, Positive Predictive Value (PPV) and Negative Predictive Values (NPV) considering the dipstick results by the investigators as the gold standard. The proportion of participants inferring the right result in the urban and rural school was individually calculated. Sensitivity was defined on the basis of ability to self screen for UTI as, the number of participants with self screened positive UTI results as a percentage of the total number of positive UTI. Specificity was defined as the number of participants with self screened negative UTI results as percentage of total number of negative UTI.
The association between UTI and DWI, holding urine and perineal hygiene was calculated individually using Chi-square tests.
Results
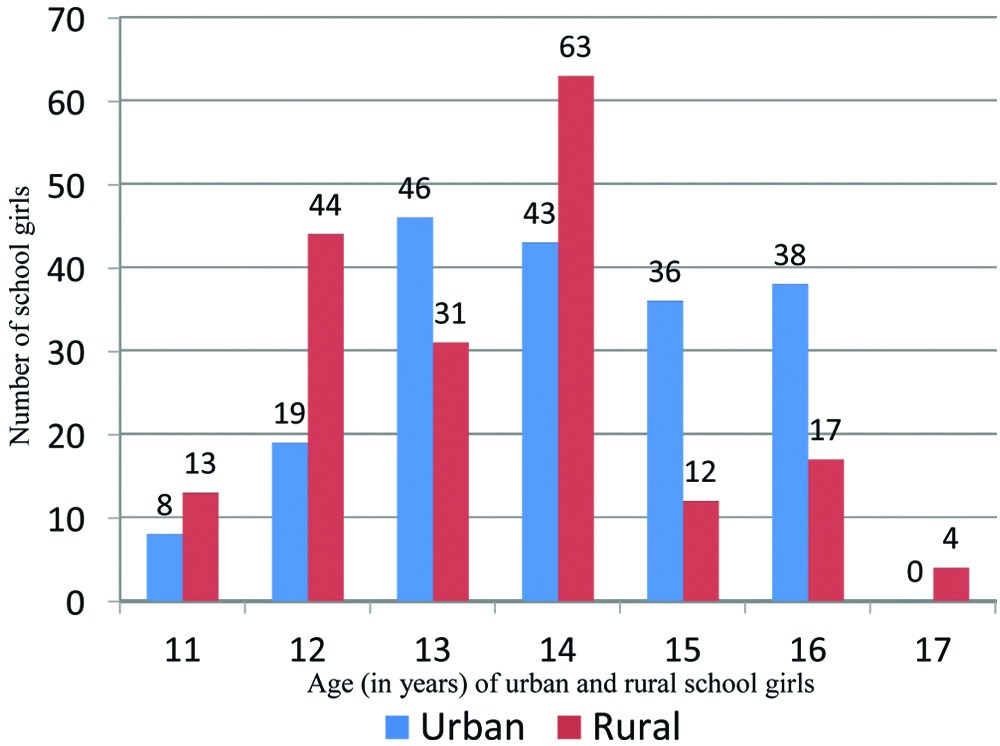
Three participants were not considered because elevated urine protein may decrease the result of Leukocyte Esterase test. In the present study 374 participants, including 184 from rural school and 190 from urban school self screened for UTI. The results of self screening and data obtained from the questionnaire were evaluated. The graph [Table/Fig-1] shows the age distribution of the participants; the median age was 14 years. The participants were asymptomatic except one rural school participant who had dysuria. Among 374 adolescent girls, 11 (2.9%) including the participant with dysuria were positive for UTI. The results of the dipstick screening test as per the investigator’s report are shown in the table [Table/Fig-2]. Among the 11 UTI positive participants, two were positive for Griess nitrite test only, three were positive for LE test only and six were positive for both the tests. The ability of the participants to rule out UTI was assessed by comparing the results of dipstick test performed by the participants with results obtained by the investigators [Table/Fig-3,4 and 5]. Total 12 (3.3%) out of 374 participants made a false report of presence of UTI. The investigators detected two instances of UTI missed by the participants. Self screening ability for UTI was found to have a sensitivity, specificity, PPV and NPV of 81.82%, 96.7%, 42.86%, and 99.43%, respectively. Out of the 184 rural students 178 (96.74%) were able to correctly test for UTI using the reagent strips provided. Out of the 190 urban students, 182 (95.79%) were able to correctly test for UTI using the reagent strips provided.
Age-wise distribution of adolescent school girls.
Results of screening tests.
| | Rural school (n=184) | Urban school (n=190) | Total (n=374) |
|---|
| UTI positive | Only nitrite | 2 | 0 | 2 |
| Only LE | 1 | 2 | 3 |
| Both | 4 | 2 | 6 |
| UTI negative | 177 | 186 | 363 |
Ability of rural school participants to interpret the dipstick test for UTI.
| Participants report | Investigators report |
|---|
| UTI positive | UTI negative |
|---|
| UTI positive | 6 | 5 |
| UTI negative | 1 | 172 |
Ability of urban school participants to interpret the dipstick test for UTI.
| Participants report | Investigators report |
|---|
| UTI positive | UTI negative |
|---|
| UTI positive | 3 | 7 |
| UTI negative | 1 | 179 |
Overall ability of participants to interpret the dipstick test for UTI.
| Participants report | Investigators report |
|---|
| UTI positive | UTI negative |
|---|
| UTI positive | 9 | 12 |
| UTI negative | 2 | 351 |
Analysis of the contributing factors for UTI [Table/Fig-6,7,8,9 and 10] shows that UTI is significantly associated with DWI, habit of holding urine, and method of perineal cleaning. It was found that 71.4% of UTI positive rural participants and 25% of UTI positive urban participants consumed less than one litre (about four glasses) of water. Data showed that 71% of UTI positive rural participants and 75% of UTI positive urban participants had the habit of holding urine. The questionnaire answered showed that 57% of UTI positive rural participants and 50% of UTI positive urban participants practiced behind-forward method of perineal cleaning. Overall, DWI of less than one litre, habit of holding urine and unhygienic way of perineal cleaning was present in 54.55%, 72.7% and 54.55% of UTI positive participants, respectively.
Risk factors for UTI in rural school participants.
| Variable | UTI |
|---|
| Present | Absent |
|---|
| Number | % | Number | % |
|---|
| Water intake |
| Less than one litre | 5 | 71.4 | 16 | 9.1 |
| More than one litre | 2 | 28.6 | 161 | 90.9 |
| Habit of holding urine |
| Present | 5 | 71 | 63 | 29 |
| Absent | 2 | 29 | 114 | 64 |
| Perineal cleaning |
| Front to back | 3 | 43 | 147 | 84 |
| Behind-Forward | 4 | 57 | 30 | 16 |
Risk factors for UTI in urban school participants.
| Variable | UTI |
|---|
| Present | Absent |
|---|
| Number | % | Number | % |
|---|
| Water intake |
| Less than one litre | 1 | 25 | 42 | 22.6 |
| More than one litre | 3 | 75 | 144 | 77.4 |
| Habit of holding urine |
| Present | 3 | 75 | 70 | 38 |
| Absent | 1 | 25 | 116 | 62 |
| Perineal cleaning |
| Front to back | 2 | 50 | 172 | 92.4 |
| Behind-Forward | 2 | 50 | 14 | 7.5 |
Relationship between water intake and UTI in the participants.
| Water intake | UTI | χ2 | p (Chi-square test) |
|---|
| Present | Absent | Total |
|---|
| Number (%) | Number (%) | Number (%) |
|---|
| Less than one litre | 6 (54.54%) | 58 (15.98%) | 64 (17.11%) | 11.1962 | <0.01 |
| More than one litre | 5 (45.46%) | 305 (84.02%) | 310 (82.89%) |
| Total | 11 (100%) | 363 (100%) | 374 (100%) |
Relationship between habit of holding urine and UTI in the participants.
| Habit of holding urine | UTI | χ2 | p (Chi-square test) |
|---|
| Present | Absent | Total |
|---|
| Number (%) | Number (%) | Number (%) |
|---|
| Present | 8 (72.72%) | 133 (36.64%) | 141 (37.7%) | 5.92 | 0.015 |
| Absent | 3 (27.28%) | 230 (63.36%) | 233 (62.3%) |
| Total | 11 (100%) | 363 (100%) | 374 (100%) |
Relationship between perineal cleaning and UTI.
| Perineal cleaning | UTI | χ2 | p (Chi-square test) |
|---|
| Present | Absent | Total |
|---|
| Number (%) | Number (%) | Number (%) |
|---|
| Front-Back | 5 (45.46%) | 319 (87.89%) | 324 (86.64%) | 16.591 | <0.01 |
| Behind-Forward | 6 (54.54%) | 44 (12.11%) | 50 (13.36%) |
| Total | 11 (100%) | 363 (100%) | 374 (100%) |
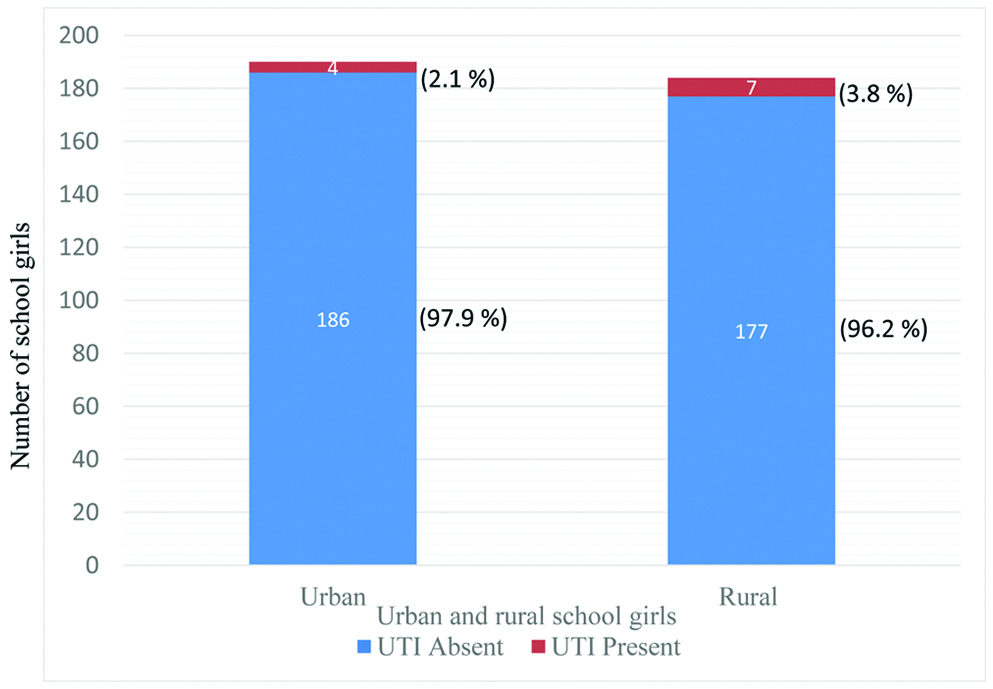
The study revealed that 7 (3.8%) out of a total of 184 adolescent girls from rural school and 4 (2.1%) out of a total of 190 adolescent girls from urban schools tested positive for UTI [Table/Fig-11].
Prevalence of UTI among urban and rural adolescent school girls.
Discussion
The necessity to recognise UTI’s before they become symptomatic underscores the importance of self screening by dipstick method. The dipstick method circumvents the limitations for routine use of urine cultures including the necessity to collect clean-voided specimen, delayed result, the burden of added cost and requirement of laboratory and expertise [1,19,24,27]. Duanngai K et al., reported self assessment for UTI by spinal cord injury patients. They observed the inter rater reliability of combined LE and nitrite dipstick tests between patients and laboratory results as moderate agreement (κ=0.52) [28]. The study for self screening for bacteriuria by Kunin CM and Degroot JE concluded that the agreement between the patient report and lab report in 146 cases was 97.3% [19]. In the present study, 96.3% of the participants were able to test correctly for UTI.
The present study protocol enabled the adolescent girls to collect a first morning specimen in the privacy of her home without the embarrassment accompanying collection at school. This also reduces the number of participants unable to produce a specimen on command [29]. The dipsticks reliability can be enhanced as in our study by taking precautions to eliminate the causes for false negative test result. The Griess nitrite test detects nitrite formed by conversion of dietary nitrate in the urine to nitrite by enteric Gram-positive bacteria when the concentration of bacteria exceeds 104 per millilitre and there is sufficient incubation time in the bladder [14,24,35]. The first-morning specimen provided overnight incubation and thus, helped the effective screening in the study.
The combined nitrite and LE dipstick test are recommended for screening UTI since the sensitivity is reported to be far superior to either test alone [14,21,23]. Similar to other studies, in the present study, dipstick was considered positive if either nitrite or LE was positive [21-23,25,26]. Specificity of dipstick for UTI is reported to be greater than microscopic urinalysis or combined dipstick and microscopic analysis [25,27]. Studies report that the dipstick testing alone provides an adequate initial UTI screen [1] with an overall NPV of 96-100% [20,22,25,26] and a negative dipstick might prevent the necessity of further urine microscopy and/or urine culture [23] which are labour intensive and consume more time than dipstick method [24,26]. In adolescent females, if diagnosis is clear routine pre treatment urine cultures are not required [5].
The age of the female adolescent students in the studies of prevention of UTI by Al-Kotb H et al., and Indhumol TD et al., were 13.6 years (mean) and 13-14 years, respectively [15,17]. In this study the median age of adolescent females was 14 years.
Hydration is important in preventing UTIs. As per WHO, Dietary Reference Intake for water in adolescent age is 2.1 to 2.3 litres/day (8-10 glasses). A study identified that majority of the adolescent girls (85.66%) consumed 2-8 glasses of water and this was one of the main contributing factors for UTI [17]. In the present study 82.89% of adolescent girls were taking more than one litre of water. Similar to our study, a strong association between DWI and UTI was reported in female nursing students [32] and adolescent girls [33]. DWI of more than one litre was observed in 20%, 28.6% (rural) and 75% (urban) of UTI positive participants in the study by Vyas S et al., and present study, respectively [32].
Complete and regular voiding of urine is an essential defence mechanism against UTI. Bacteria in the bladder multiply rapidly, so it is important not to hold urine for extended time periods. Since the description of “non neurogenic, neurogenic bladder syndrome” by Hinman F, in the 1970’s, it is acknowledged that abnormal voiding habits leads to UTI and renal damage in children with “normal” anatomy [1,36]. Similar to our study, a significant association between UTI and habit of holding urine is observed in the literature [32]. In the study of predictors of UTI among unmarried female nursing students, 60% of the UTI positive participants had the habit of holding urine [32]. In the present study, this habit was more among both the rural (71.4%) and urban (75%) UTI positive participants. Apprehension of catching germs from the public toilet seat among girls leads to limiting water intake so as to prevent the need of using the toilet; hovering over the seat leads to residual urine which promotes UTI [32,37].
In the present study the association between practice of improper perineal washing and occurrence of UTI is significant and studies have substantiated it [12,15,33]. The students have to be educated upon the right methodology to clean themselves after defecation. When washed from back to front after using the toilet, Escherichia coli in the perineal skin can spread and enter the urinary tract through the urethra [38]. In the present study, improper perineal washing observed in the urban setting (8.42%) was comparable with that reported in the literature (9.4%) [15]; however, among the rural participants it was found to be notably higher (18.48%).
The significant association between UTI and contributory risk factors among adolescent girls underscores the need for improving their knowledge about the genitourinary tract physiology, reasons for UTI, appropriate health practices and benefits of self screening. As compared to the literature the poorer perineal hygiene among rural participants observed in the present study is possibly due to lack of awareness. Studies report that structured teaching programme produced significant improvement in the knowledge of adolescent girls about UTI and its prevention [15,17].
Prevalence of UTI among children is observed to differ with socioeconomic status, geographical areas [23,39], age, gender and selected population. In the present study two selected populations differing in socioeconomic status were studied; the prevalence of UTI was found to be 3.8% and 2.1%, respectively in rural and urban settings. In children, Antwi S et al., Asscher AW et al., and Mohammed A et al., reported prevalence of 2.1%, 2.8% (11 years age) and 6% respectively with predominance in girls than boys [23,29,39]. A Nigerian study reported a prevalence rate of 11.96% among children and adolescents [13]. In adolescent girls, the incidence of UTI was reported as 9.1% [33] and 12.7% [12].
Limitation
Although dipstick nitrite detects all the Gram-negative (most common causative) UTI and some Gram-positive UTI, infections with nitrate reductase-negative organisms would be missed. Though we gave clear instructions for the dipstick positive cases to seek further confirmation and appropriate treatment, we have not done a follow-up with which a better data regarding reliability of dipstick method, association of UTI with contributory factors and prevalence of UTI could be established. The contributory factors to UTI like menstrual hygiene, vaginal discharge and nutrition are not assessed.
Conclusion
The high ability of students to self test in the present study reinforces the feasibility of implementing regular self screening for UTI as an expanded programme of health education in schools. In this regard the combination of nitrite and LE reagent dipstick analysis of urine is a rapid, reliable, cost effective and convenient preventive measure for UTI especially in rural areas where laboratory facility for urine microscopy and culture are not readily available. The programme of self screening for UTI is not another subject in school but a life skill which can be used at home and in their community to identify asymptomatic UTI, thereby preventing its long-term sequelae.