IDA is a common background jeopardy identified for many adverse outcomes in pregnancy. Reproductive age women have high prevalence of IDA (30%) globally [1]. In India, prevalence of anaemia in adult women is 53% as per National Family Health Survey 2015-2016 [2]. IDA is most common during antenatal period with an incidence of 60% in urban and 69% in rural population of India [3]. IDA has been identified to be responsible for over 40% percent of maternal mortality and morbidity. Therefore, regular surveillance, prophylactic therapy, dietary guidance and treatment are necessary before, during and after pregnancy. Anaemia is prevalent despite the recommended universal iron supplementation after 12 weeks of gestation. This is due to multiple factors such as noncompliance to oral iron therapy, depleted iron stores, deficient nutrition, worm infestations etc. Moderate anaemia during pregnancy especially during the second trimester is often treated by IVIS and its safety and efficacy is well established. In a retrospective analysis Haldar P et al., demonstrated that IVIS increases mean Hb by 1.76 g/dL when 400 mg was given to pregnant women with moderate to severe anaemia [4]. In a case control study conducted by Raut SV and Biniwale PA, they concluded that IVIS was safe in IDA anaemia during pregnancy [5].
In clinical practice, management of severe anaemia in pregnancy with haemoglobin less than 7 gm/dL is challenging [6]. Swift improvement in the iron deficient state which would benefit the mother and result in better foetal outcome through improved placental perfusion is desired. Additionally, iron stores and oxygen carrying capacity should be optimised to overcome the losses during delivery and for healing during post-partum period. For this safe and effective modality is essential. Till now, there is no head to head trial comparing IVIS with PCV for severe IDA in pregnancy. Keeping this in mind, this prospective study was planned to offer IVIS to treat severe anaemia during pregnancy and to compare it with current standard treatment i.e., PCV for safety and efficacy.
Materials and Methods
This randomised, open label study was conducted during January 2017-December 2018 and 30 pregnant women between gestational age of 13-32 weeks with haemoglobin between 4-7 gm% were enrolled. Women with haemoglobinopathies, severe concurrent illness (cardiovascular, renal, hepatic or any other systemic diseases) as determined by clinical examination, history of chronic inflammations and known hypersensitivity to iron preparations were excluded. Ethical clearance was obtained from Institutional Ethical Committee (Registration No. ECR/313/Inst/MH/2013/RR-16) and written informed consent was obtained from the participants and nature of study was explained to them.
Women with severe anaemia were admitted in the tertiary care hospital for investigations and therapy. All the investigations to classify anaemia and identify other causes such as sickle cell disease, thalassaemia, iron stores etc., were undertaken. Patients were randomised by a computer-generated random number table method to undergo either IVIS or PCV therapy. Both dosages were calculated as per the deficit. The deficit is haemoglobin level of the mother at the time of recruitment minus 12 g/dL taken as endpoint. The iron sucrose dose was administered as per the standard formula:
Amount of iron deficit (mg)=Pre-pregnancy Body wt. (Kg)×Hb deficit in gm %×2.4+500 [7]
The number of PCVs was decided based on the fact that 1 PCV rise the haemoglobin level by 1 g/dL [8]. Patients were hospitalised during the primary treatment as both iron sucrose as well as PCV were administered on alternate days i.e., dose of Iron sucrose-100/200 mg in 100 mL NS infused over a period of 20 minutes (alternate day) and PCV-one unit administered over 1 hour. Patients were advised to follow-up on day 8 and then report for regular follow-ups till delivery. Haemoglobin level, MCV, reticulocyte count was done on day 8, 15, 30 and at delivery in both the groups while serum ferritin levels were done on day 30 and at delivery. Primary endpoint for treatment efficacy was considered to be rise in Haemoglobin% by 2 gm% for both the groups. Secondary end points were rise in serum ferritin levels, improvement in RBC indices (MCV and reticulocyte count), and improvement in symptoms of anaemia.
Adverse reactions, both minor and major, were noted. Patients were counselled about the effects of anaemia, the treatment methods and regular follow-up. They were given nutritional advice for iron rich foods and were offered Albendazole 400 mg as a single dose anthelminthic treatment.
Statistical Analysis
Data were presented as number (%) or mean±SD/median (min-max) as appropriate. Baseline categorical variables were compared between the groups using Chi-square test and continuous variables were compared using Student’s t-test/Repeated Measures ANOVA test followed by Dunnett’s test. All statistical analysis was carried out using IBM SPSS statistics version 25.
Results
A total of 30 pregnant women satisfying the inclusion and exclusion criteria were enrolled in the study. These patients were then assigned to the treatment groups according to randomisation plan. As such 15 cases were allotted to Group IVIS and Group PCV each. Due to noncompliance and haemoglobinopathies, 4 patients were withdrawn from the study. After the completion of the study, a total of 26 cases were analysed.
There were four dropouts before analysis. Three cases, during routine screening for IDA were diagnosed for the first time to have thalassaemia minor. One case in Group IVIS, required reinfusion was excluded [Table/Fig-1]. However, these patients were duly followed-up till delivery, and were given requisite medical care.
Disposition of participants.
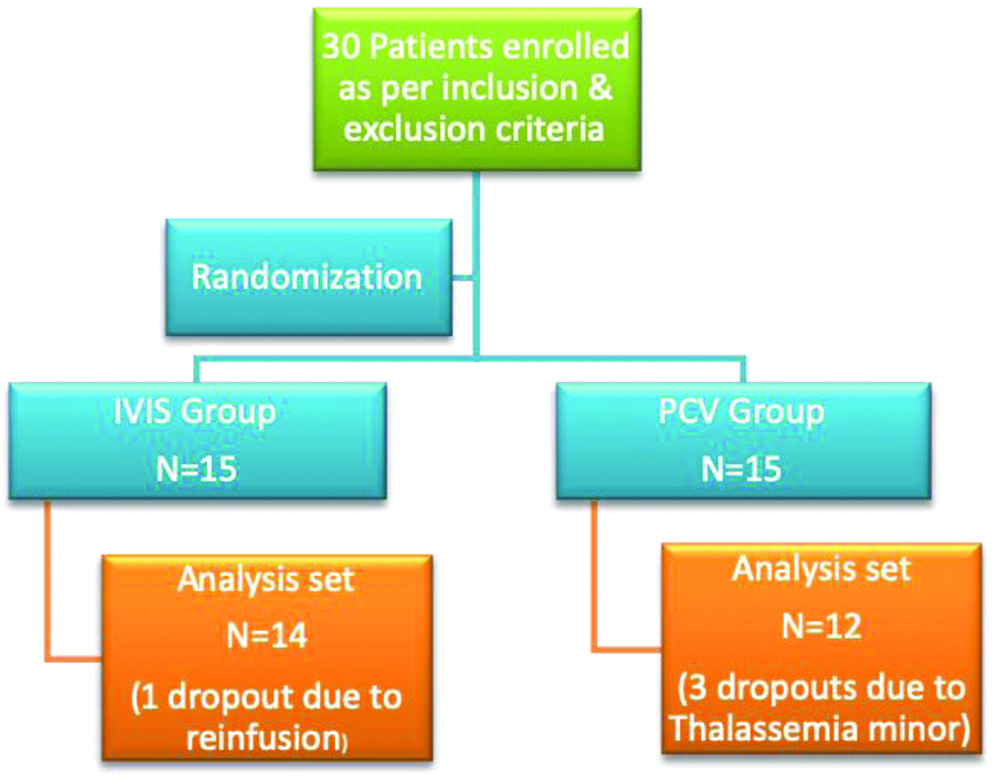
A total of 26 patients data were evaluated for efficacy and safety. This included 14 cases in Group IVIS and 12 cases in Group PCV [Table/Fig-1]. All the subjects were followed-up till confinement. Both the groups were comparable in basal demographic characteristics viz., mean age, weight at the time of recruitment [Table/Fig-2].
Baseline characteristics.
| Group IVIS | Group PCV |
|---|
| Age (Y) (Mean±SD) | 22.85±4.24 | 24.42±2.87 |
| Range | 18-26 | 22-26 |
| Weight (kg) (Mean±SD) | 47.53±3.40 | 44.25±7.47 |
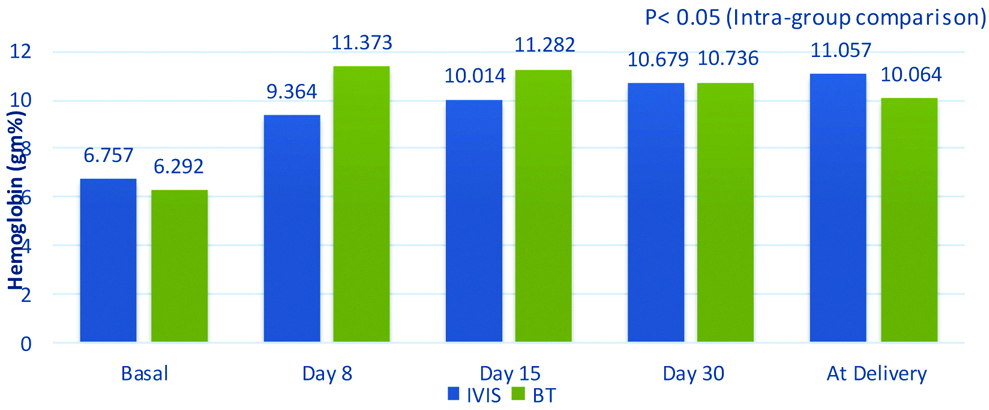
There was significant rise in haemoglobin on Day 8 from the baseline (2.607 gm% in Group IVIS and 5.081 gm% in Group PCV; p<0.05), while the day 30 levels were similar for both viz., 3.9 gm% for Group IVIS and 4.4 gm% for Group PCV respectively. Mean rise of haemoglobin at the end of the study (delivery day), over the basal values was 4.3 gm% in IVIS Group, and 3.772 gm% in PCV Group [Table/Fig-3].
Comparative haemoglobin values (Mean).
IVIS: Intravenous iron sucrose; BT: Blood transfusion
Using Repeated Measures ANOVA test followed by Dunnett’s test
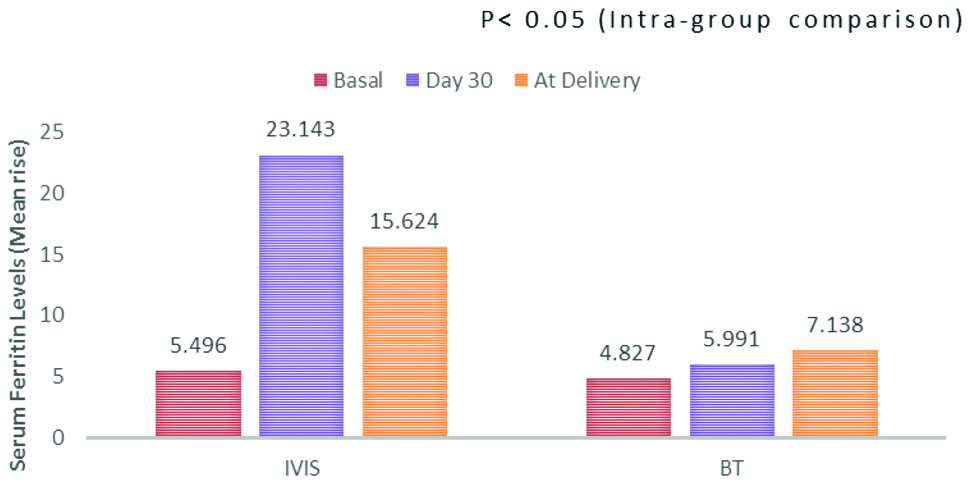
Significant rise in serum ferritin from the basal value was found on day 30 (mean rise of 17.647 ng/mL; p<0.05) and delivery day (mean rise of 10.128 ng/mL; p<0.05) in Group IVIS. In the Group PCV day 30 (mean rise of 1.163 ng/mL) and delivery day (mean rise of 2.30 ng/mL) [Table/Fig-4].
Mean rise of serum ferritin levels.
IVIS: Intravenous iron sucrose; BT: Blood transfusion
Using Repeated Measures ANOVA test followed by Dunnett’s test
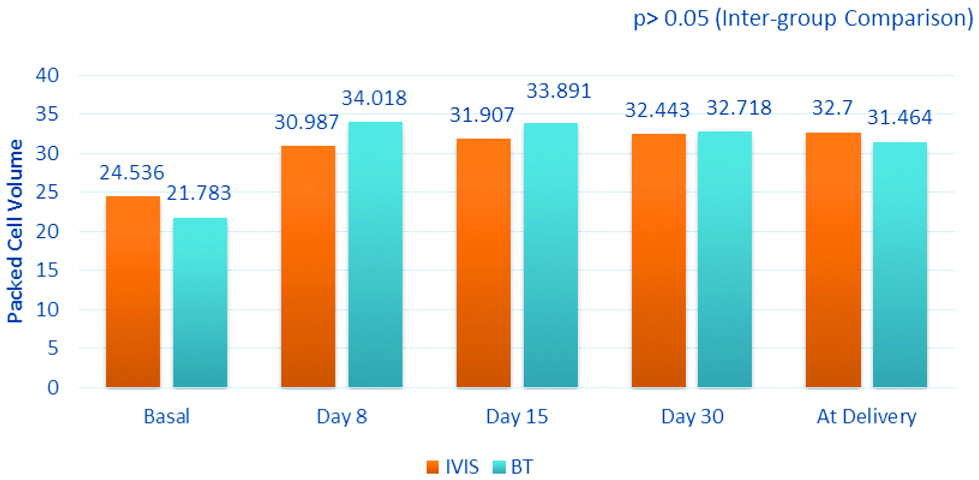
The mean rise in the PCV from the basal values to that of the delivery day was found to be 8.164% and 9.681% for Group IVIS and Group PCV respectively [Table/Fig-5].
Comparative packed cell volume values (Mean).
IVIS: Intravenous Iron Sucrose; BT: Blood Transfusion
The baseline values of reticulocyte count were observed to be comparable in both the groups at the time of recruitment. A progressive fall was found in the values of reticulocyte count at all the follow-up visits with mean values of 0.622% and 0.672% in Group IVIS and Group PCV respectively and was comparable at all the follow-up visits.
Compliance and Safety Evaluation
Compliance for study was monitored by telephonic follow-up. All the patients were monitored for any adverse reactions. Patients recruited in IVIS group were monitored for any adverse reaction during IVIS transfusion. Before giving the total infusion dose, a test dose was given, to check hypersensitivity reactions. Apart from minor reaction like thrombophlebitis (01), no major reaction was noted. Patients recruited in Group PCV were also monitored for any transfusion reaction. Of all the patients recruited, only one had transfusion reaction which required termination of treatment.
Discussion
Severe IDA is associated with an increased risk of maternal deaths and serious adverse pregnancy outcomes such as sepsis, susceptibility to infections, preterm deliveries, need for cesarean delivery [9,10] and possibility of NICU admission for the newborn. Severe IDA has several deleterious effects on the mother as well as the offspring especially babies born with anaemia [11].
Blood transfusion and iron sucrose has different pharmacokinetic properties. Iron sucrose belongs to the iron complexes of the half robust and medium strong type (molecular mass between 30,000 and 100,000 Da), which after intra venous administration, deliver the complexed iron from the serum to endogenous iron-binding proteins with a half-life of 90 minutes [12]. It is taken up mainly by the reticuloendothelial system of the liver as well as by transferrin and apoferritin, the spleen and the bone marrow. Subsequently, it is rapidly metabolised and readily available for erythropoiesis giving a lasting effect for haemoglobin maintenance [12]. On the other hand, increased PCV can cause adverse effects overloading an existing hyperkinetic circulation in a pregnant anaemic woman. It is known that reduced oxygen carry decreased haemoglobin compensated by increase in cardiac output, redistribution of blood flow, increase in the 2,3 DGP content of the blood cells, in order to effectively decrease oxygen to the tissue circulation. Even normovolaemic patient have, transient rise in venous pressure which continues to rise for several hours even after transfusion. In a pregnant woman PCV can cause further rise in blood volume and thus cardiac overload [13]. Hence, if transfusion is considered for severe anaemia in a pregnant woman, the advantage of raising the arterial oxygen content has to be weighed against the hazards of overloading an already hyperkinetic circulation. This is where, one can find IVIS to be beneficial over blood transfusion. Kriplani A et al., conducted a prospective study on IVIS therapy for moderate to severe anaemia in pregnancy. Showed that parenteral iron therapy using iron sucrose was effective in increasing haemoglobin (from 7.63±0.61 to 11.20±0.73 g%, p<0.001), serum ferritin (from 11.2±4.7 to 69±23.1 μg/L, p<0.001) and other haematological parameters in pregnant women with moderate anaemia [14]. A prospective interventional study conducted by Tembhare A et al., aimed to discern the efficacy of IVIS in the management of IDA during pregnancy. This study included pregnant women with gestational age more than 20 weeks with IDA. In the results, comparison of IVIS with oral iron showed significant improvement in haemoglobin and serum ferritin on day 30 (p=0.0001) who received IVIS [15]. A prospective study was conducted by Vijayasree M to determine efficacy of IVIS in pregnant women with IDA presenting at tertiary care hospital. Results showed significant improvement in the haemoglobin of the patients who received IVIS. The target of 11 gm% haemoglobin was achieved by the patients and iron sucrose was safe and well tolerated [16].
In the present study, it was observed that IVIS produced progressive and a comparable rise in haemoglobin concentration when compared to blood transfusion. Maternal iron stores were restored more rapidly with intravenously administered iron than blood transfusion. Iron sucrose was well tolerated with no serious adverse effects where as in blood transfusion about 6.33% patients had blood transfusion reaction requiring termination of treatment. IVIS has lower incidence of adverse allergic reactions [9]. In a retrospective study including 50 pregnant patients showed significant improvement in the various haematological parameters in IVIS group. There were no significant allergic reactions with iron sucrose. These results were comparable with our study results [17]. In a prospective, randomised controlled trial Neeru S et al., demonstrated that IVIS group had no major side-effects for the treatment of anaemia in pregnancy [18].
Limitation
In this study, we did not include post-delivery follow-up which could have further added valuable data on post-partum outcome.
Conclusion
Intravenous iron therapy is a safe alternative for the treatment of anaemia, being able to reduce the need of blood transfusions with its concomitant side effect such as anaphylactic shock, febrile and haemolytic reactions, transmissible infections (hepatitis B and C, HIV, protozoan and bacterial), alloimmunization and graft versus host disease. In present study, IVIS appeared to provide a rapid replenishment of both iron stores and haemoglobin level for women with severe IDA in pregnancy when compared to blood transfusion. However, a larger study is needed to examine the risks of the infusion and the accompanying clinical benefits this may provide.