Modern lifestyle, unhealthy food habits, digestive tract pathology and stress are the identified risk factors associated with many digestive diseases such as GERD, acidity or the peptic ulcer. Gastro-esophageal Reflux (GER) may be described as a normal, post-prandial and physiologic retrograde flow of gastric contents into the oesophagus [1]. GERD not only has the local effects confined to the esophagus but has also been observed associated with the presence of DE with loss of surface enamel or dentin due to gastric acid reflux [1].
GERD has shown various oro-dental ailments and the most common is DE. DE may be caused due to exposure to intrinsic or extrinsic acid. The extrinsic factors include excessive, regular consumption of acidic food or beverages, acidic sports drink intake or chewing Vitamin C tablet whereas; intrinsic factors associated with GERD are acid regurgitation, recurrent stress-induced vomiting or excessive alcohol consumption etc., [2-5]. Erosion due to intrinsic acids is also evident in women addicted to induce vomiting as in bulimia or anorexia nervosa and rumination [4,5]. Other oral manifestations of GERD may include; halitosis, burning sensation with ulceration of oral mucosa, dysgeusia, xerostomia or sometimes with increased salivation [6]. A study carried out in GERD patients revealed that the prevalence of GERD in patients less than 20 years was 4.4%, whereas it was 11.6% in adults [7]. DE may cause loss of enamel and dentin, and in advance stages may cause hypersensitivity, pulp exposure and loss of tooth form. Thus, this cross-sectional study was planned to evaluate the prevalence and severity of DE.
Materials and Methods
A cross-sectional study was conducted in a Tertiary care hospital to evaluate the prevalence and severity of DE. In the present study 100 patients diagnosed with GERD and 100 patients from the Out-patient Department (OPD) were examined from January 2018 to May 2019. Ethical approval was obtained from the institutional Local Ethical Committee for the study (GDCH/Ethical committee/Project/1085-86/08). Using Roff formula, with average population size 134 (average OPD), confidence level 95% and confidence interval 9.8; 100 patients were selected at random by a team of restorative dentists in each group. Patient’s personal information including name, age, gender, residence, qualification and occupation etc., was noted for record purpose. For the study, patients diagnosed with GERD having a history of frequent gastric refluxes atleast for one year with or without history of vomiting; 2 or more times a week or history of peptic ulcer due to GERD were preferably selected. Written informed consent was obtained from all voluntary participants.
A structured case history questionnaire related to gastric reflux frequency, vomiting frequency, its duration, dietary habits, medicinal and other related habits contributing to DE etc., were also recorded. The pattern of DE was recorded and the severity of DE was measured using Lussi’s Erosion index as it is simple, reliable and reproducible indexing system [8,9]. According to this index, Grade I, DE (Mild) denotes loss of enamel, rounded cusps and incisal grooving, Grade II (Moderate) denotes DE extended into dentin in about half of tooth surface whereas; Grade III (Severe) indicates DE extended into dentin involving more than half of tooth surface.
Statistical Analysis
Data obtained was compiled on MS Office Excel Sheet (v. 2010) and subjected to statistical analysis using Statistical package for social sciences (SPSS v 21.0, IBM). Descriptive statistics like mean age, gender-wise distribution (overall and group-wise) has been depicted. Comparison of frequencies of subjects with erosion, maximum erosion severity, affecting tooth tissue (enamel or dentin) with the control group and teeth sensitivity was done using Chi-square test. For all the statistical tests, p<0.05 was considered to be statistically significant, keeping α error at 5% and β error at 20%, thus giving power to the study as 80%.
Results
Out of 100 GERD patients, 57 (57%) were males and 43 (43%) were females; whereas in control group 62 were males and 38 were female participants [Table/Fig-1].
Gender and Age-wise distribution of participants.
| Groups | n=100 Patients/Group | Minimum (in years) | Maximum (in years) | Mean (in years) | Std. deviation |
|---|
| M | F |
|---|
| GERD patients | 57 | 43 | 20 | 60 | 41.65 | 13.295 |
| OPD patients (Control group) | 62 | 38 | 20 | 60 | 37.21 | 12.637 |
Total of 88 (88%) participants of GERD group has shown the presence of DE of a varying degree from Grade I to Grade III whereas; only 47 (47%) showed DE in control group patients (Chi-square value=28.408, df=10, p=0.0002). Results also showed that Grade I is the most common type of erosion which was observed in 54 (54%) patients with GERD and 45 (45%) patients in the control group [Table/Fig-2,3].
Dental erosion severity grades.
| Erosion grading | Grade I (Mild) | Grade II (Moderate) | Grade III | Total |
|---|
| In GERD patients | 54 | 32 | 2 | 88 |
| In OPD patients (Control group) | 45 | 2 | 0 | 47 |
Chi-square value=28.408, df=10, p=0.002
Grade-wise distribution of patients (Grade I/Grade II/Grade III).
| Affecting tooth tissue (Grade I/Grade II/Grade III) | Total |
|---|
| No erosion | Grade I | Grade II | Grade I + Grade II | Grade I + Grade II+Grade III |
|---|
| GERD patients | 12 | 54 | 15 | 17 | 2 | 100 |
| OPD patients | 53 | 45 | 2 | 0 | 0 | 100 |
Chi-square value=174.68, df=20, p<0.001
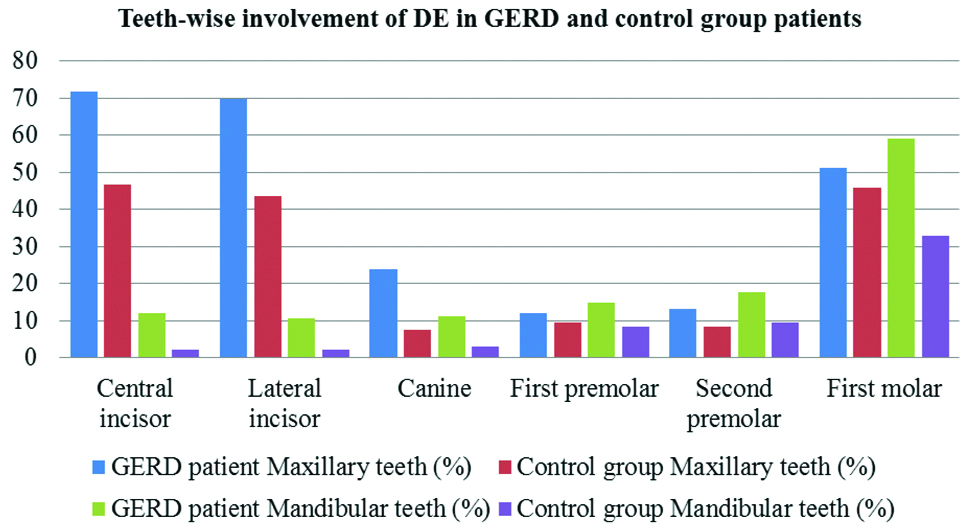
It was also observed that the teeth of both the arches were affected by erosion with more or less severity. Dentinal sensitivity was also observed in 68 (68%) GERD patients and 25 (25%) in the (OPD patients) control group [Table/Fig-4]. In GERD group, the prevalence of DE in maxillary and mandibular teeth showed that 71.59% of the maxillary anterior teeth had erosion whereas; 59.09% of mandibular posterior teeth showed DE which is more than their anterior counterparts. It was also observed that the lingual surfaces of mandibular anterior teeth were affected the least by DE with the prevalence of 2.27%.
| Teeth sensitivity |
|---|
| Group | Absent | Present | Total patients |
|---|
| GERD patients | 32 | 68 | 100 |
| OPD patients | 75 | 25 | 100 |
Chi square value=48.396, df=5, p<0.001
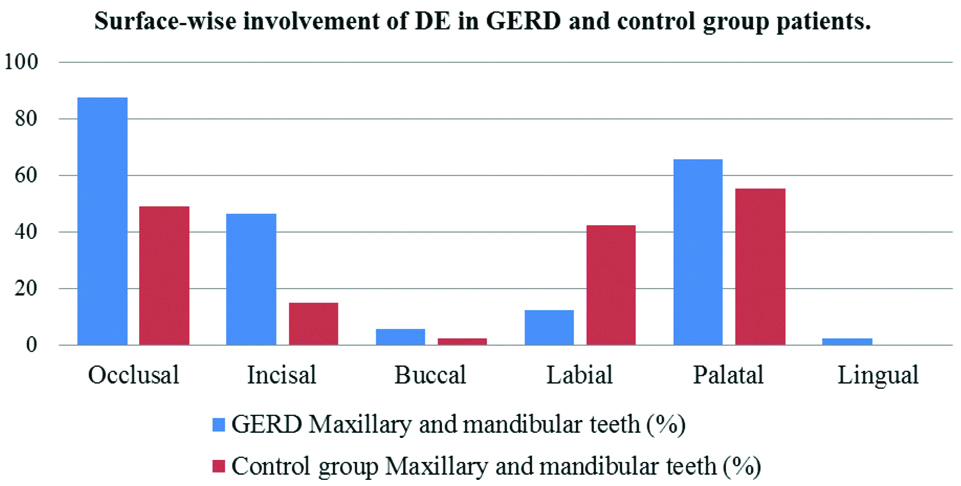
In the control group, the maxillary anterior and posterior teeth have the same prevalence of DE in 46.81% teeth whereas; amongst mandibular teeth, 31.14% molars showed DE. The palatal surfaces of maxillary anteriors showed maximum erosion in 55.26% teeth whereas; occlusal surfaces of mandibular posterior teeth were more commonly affected in 40.43% teeth. Lingual surfaces of mandibular anterior teeth were unaffected by tooth erosion. In both the groups, the teeth showed concurrent evidence of varying grades of severity of DE and it had affected different surfaces of the majority of teeth [Table/Fig-5,6].
Teeth-wise involvement of DE in GERD and OPD patients.
Surface-wise involvement of DE in GERD and OPD patients.
Discussion
GERD is considered as a common dental disease attributed to modern lifestyle and unhealthy food habits. Digestive acids of endogenous origin contain hydrochloric acid which is produced by parietal cells; generating pH of 2-3 [10]. This pH is more acidic than exogenous acids which not only causes DE but also affects the inner mucosal lining of the oropharynx, oesophagus or the respiratory system [11-15]. Studies in the literature showed that the frequency of GERD significantly increases after the age of 40 years and there is a wide disparity in its prevalence [2,3,6,16].
Various in-vitro and in-vivo clinical studies have been carried out which showed assertive relationship between GERD and DE in children and adults [Table/Fig-7] [7,17-26]. An evidence-based Montreal consensus was carried out in 2006 in which 44 doctors around the globe confirmed by voting that in patients diagnosed with GERD the occurrence of DE has increased [3].
Prevalence of dental erosion in various studies [7,17-26].
| Sl. no. | Author | Year | Studied population | Prevalence |
|---|
| 1 | Jarvinen V et al., [17] | 1988 | 109 | 20% |
| 2 | Firouzei MS et al., [18] | 2011 | 15 | 87% |
| 3 | Meurman JH et al., [19] | 1994 | 117 | 24% |
| 4 | Gudmundsson K et al., [20] | 1995 | 14 | 21% |
| 5 | Bartlett DW et al., [21] | 1996 | 36 | 64% |
| 6 | Loffeld RJ [22] | 1996 | 293 | 32.5% |
| 7 | Gregory-Head BL et al., [23] | 2000 | 20 | 50% |
| 8 | Dahshan A et al., [24] | 2002 | 24/37 | 83% |
| 9 | Munoz JV et al., [25] | 2003 | 181 | 47.5% |
| 10 | Oginni AO et al., [26] | 2005 | 125 | 16% |
| 11 | Okimoto E et al., [7] | 2015 | 1859 | 4.4% in young patients 11.6% in adults |
Pace F et al., in their systematic review evaluated 17 different observational and case-control studies and concluded 24% as an average incidence of DE in patients with GERD. They also observed the prevalence of GERD with DE in children and adults as 17% and 32.5% respectively [27]. Tolia V et al., in their systematic review found a higher prevalence of DE in children diagnosed with GERD [14].
Bartlett DW et al., in their study observed that 23 (64%) out of 36 patients had significant palatal erosion with a history of severe GER. They have also concluded that all these patients were ‘silent refluxer’ and the regurgitation of gastric secretion in GER causes palatal erosion [21]. In other studies, it was evident that in patients with GERD; the prevalence of DE is 6%-10% [28,29].
Meurman JH et al., examined 117 patients with GERD and observed dental erosion in 28 (24%) patients of the studied population [19]. In a different study by Muñoz JV et al., compared the prevalence of dental erosion over 2 years in 253 individuals; out of which 181 patients had GERD and 72 were the healthy volunteers. Results of the study showed that regardless of age, the prevalence of dental erosion was significantly higher and severe in the GERD group. Investigators also concluded the DE as an extra-esophageal manifestation of GERD [25].
Lussi A et al., in the study observed that; 42.6% of Swiss adults between the age of 46-50 years; had an average of 3.9 teeth affected by erosion and having atleast one tooth with severe erosion observed on the occlusal surface [8]. Jarvinen V et al., observed that 20% of patients out of 109 patients having gastro-oesophageal symptoms had DE [17].
In another study by Jones RR et al., DE was observed in 69% of bulimic female patients causing exposure of enamel and dentin due to the acidic gastric juices. DE was observed on the palatal surfaces of maxillary teeth and the facial surfaces of the central incisors and canines in 50% and 70% of the patients respectively [30]. A comparative study was done by Bartlett DW et al., to compare the erosive effect of gastric juice and a carbonated drink on the enamel and dentine and concluded that gastric juice (pH 2.5) has a greater potential to cause erosion than a carbonated drink [31].
From the observations in the present study, a conclusion regarding the prevalence of tooth erosion can be drawn that high prevalence of DE can be due to changing lifestyles and dietary habits which lead to chronic gastric disturbances resulting in chronic hyper-acidity. In developing Asian countries like India; spices and oils are essential parts of regular meals. Similarly, due to routine intake of such food and unscheduled or skipped meals may also lead to gastritis; while in western countries, DE might be due to anorexia nervosa, bulimia, habitual intake of acidic beverages or alcohol etc.,
In this study, out of 100 patients examined, only 5 (5%) had a peptic ulcer. Also, out of 100 GERD patients, 54% showed mild erosion, 32% showed moderate erosion and 2% showed severe DE. This severity has been essentially related to the high acidic pH (around 1.0-2.5) of gastric reflux or the vomitus. Such a low pH is capable of DE resulting in dentin exposure earlier and with a greater severity than the other acid sources.
Similar results were observed in the present study as in the literature about the prevalence of DE in GERD patients [18,21,24]. A probable explanation thus can be established regarding the surfaces affected by DE that, the palatal surfaces of maxillary anteriors and the occlusal surfaces of maxillary molars were in direct contact with gastric reflux or the vomitus for a longer time. Thus, this site-specificity and severity for DE have been attributed to the acid reflux exposure and less cleaning activity by saliva and the tongue. Lingual surfaces showed least erosion and it relates to the continuous cleansing action of the tongue and the saliva.
Clinically, the dental clinician must not overlook the importance of dietary history with other medicinal or other contributing history while diagnosing and treating DE. Also, DE has similar clinical features with other wearing diseases of teeth and it causes irreversible dissolution of enamel which may lead to loss of vertical height, loss of aesthetics and dentinal sensitivity. Thus, the operator must rule out the causative factors and directs the management towards its prevention, early diagnosis and treatment and regular follow-up to prevent the extensive loss of tooth structure. Further studies are recommended to evaluate effect of multifactorial origin of DE in a large population.
Limitation
DE is a known multifactorial disease where GERD is a prime intrinsic factor identified in its causation. In the present study, randomly selected patients with diagnosed GERD were evaluated as the cause of the DE; while the other extrinsic factors concurrently responsible for the erosion, could not be isolated. Thus, absolute role of GERD on dentition exhibiting as DE could not be established exclusively.
Conclusion
DE might be considered as a connotative feature of the patient diagnosed with GERD with its high prevalence. It can be concluded that severity of DE could be dependent on pH of the acid reflux, its duration of contact with the teeth as well as the time of its oral clearance after acid reflux or vomiting. The erosive loss of teeth may be severe if GERD has not been diagnosed and treated promptly. Thus, prevention is the key in the successful management of DE.
Chi-square value=28.408, df=10, p=0.002
Chi-square value=174.68, df=20, p<0.001
Chi square value=48.396, df=5, p<0.001