Bilirubin Encephalopathy (BE), a complication of hyperbilirubinaemia is largely preventable, and continues to be a growing concern especially in resource-limited settings amongst neonates with undetected, neglected or poorly treated hyperbilirubinaemia [1]. It results in significant morbidity characterised by ABE, and later, kernicterus in the form of auditory neuropathy and chorio-athetoid cerebral palsy; and mortality [2]. In Nigeria, the prevalence of BE ranges from 2.3% to 15.9% [3-5].
The term Bilirubin-Induced Neurologic Dysfunction (BIND) was suggested by Johnson L et al., [6] to describe the changes associated with BE. They proposed a scoring system to quantify the severity (subtle, moderate, advanced), onset, progression of the clinical manifestation and risk of developing encephalopathy [6]. In South western Nigeria, [5] the usefulness of the modified-BIND score (a modification of the original BIND score) was assessed and it was reported to have a sensitivity of 90.7%, specificity of 97.7%, positive predictive value of 88.9%, and negative predictive value of 98.2% in identifying neonates with ABE.
BAER otherwise called ABR is a non-invasive, highly sensitive and highly specific screening tool [7] used for identifying impending encephalopathy in neonates with hyperbilirubinaemia; it identifies subtle findings that could be reversed and detects early hearing abnormalities [8,9]. It has been reported as an effective new-born hearing screening tool especially in severe hyperbilirubinaemia requiring exchange transfusion [9,10]. It is recognised as the most objective method of evaluating the auditory neural pathway in neonates and infants [11,12]. Auditory neuronal pathway is reported to be the most sensitive to bilirubin toxicity, that manifest as Sensori-Neural Hearing Loss (SNHL) [2,9]. Therefore, evaluation of the auditory pathway may improve the identification of bilirubin induced neuro toxicity in the neonate [13,14]. Auditory Neuropathy Spectrum Disorder (ANSD), a type of SNHL is one of the earliest features that may be seen even in the absence of clinical manifestations of BE [13,15], accounting for 7% of permanent childhood hearing loss [16]. The finding of high incidence of SNHL amongst neonates with hyperbilirubinaemia ranging between 25.7% and 56.7% [17-25] is of great public health significance considering the incidence of SNHL at near epidemic proportions in some developing countries [26-28].
ABE is associated with significant morbidity and mortality, hence early and accurate detection is important. The need to predict and prevent it using the most precise tool becomes absolutely necessary. Autopsy is one of the best ways to confirm the diagnosis of BE, but it is crucial to identify such a disorder before it results in mortality, as such the need to rely on ancillary tests like the BIND score and BAER becomes essential in clinical practice.
This study was designed to determine if the BAER and BIND score adequately identify neonates with ABE, and to establish the effectiveness of the BIND score when compared with BAER in detecting these babies with encephalopathy. This will be of relevance especially in resource poor settings where facilities for BAER may be lacking. There is no data exploring the relationship between BIND score and BAER in detecting ABE, with the former expected to be preferable in resource-limited settings because it can be administered within the routine physical examination without additional cost.
Materials and Methods
A cross-sectional descriptive study was conducted between September and December 2015 in Aminu Kano Teaching Hospital (AKTH), a tertiary health institution in Kano, Nigeria. It was approved by the research and ethics committee (NHRCE/21/08/2008/AKTH/EC/1308). Written consent were obtained from the parents/caregivers of the neonates before enrolment.
Study Population
Neonates with significant hyperbilirubinaemia (TSB >95th percentile for age in hours [11]) were enrolled as cases during the study period.
Healthy age and sex matched neonates attending immunisation clinic for Bacille Calmette-Guerin (BCG) vaccination were enrolled as controls.
Inclusion Criteria (Cases)
Term neonates with TSB of ≥10 mg/dL.
Neonates with BIND score (1-9) irrespective of the TSB level.
Inclusion Criteria (Controls)
Healthy age and sex matched neonates
Exclusion Criteria
Prematurity, intrauterine growth restricted babies and neonates with the following abnormalities: suspected chromosomal disorders, cranio-facial malformations, birth asphyxia, sepsis, meningitis, hypoglycaemia family history of congenital hearing loss and congenital infections. All were excluded because they may be confounders and can present with BAER abnormality on its own even without hyperbilirubinaemia.
Sample Size Determination
The minimum sample size per group of neonates required was estimated using the formula:
n=(Za+Zb)2 (p1q2+p1q2)/(p2-p1)2Where,
n=Minimum sample size required
Za=1.96, the standard normal deviate corresponding to 5% level of significance.
Zb=0.84, the standard normal deviate corresponding to the power of the test to detect differences, set at 80%.
P1=38% (0.38), the population prevalence of significant neonatal hyperbilirubinaemia [29].
q1=complementary probability to p1=1-P1=0.62
P2=16.8% (0.168), the population prevalence of neonatal hyperbilirubinaemia obtained by Coulter J [30].
q2=complementary probability to p2=1-P2=0.832
Therefore, the minimum sample size, n=66 was used for each group.
Sampling Method
Every consecutive neonate that presented with significant hyperbilirubinaemia and who met the inclusion criteria was enrolled until the sample size was reached. Age and sex matched neonates from BCG immunisation clinic who met the inclusion criteria were also enrolled simultaneously.
Data Collection Technique
Each neonate was examined at presentation. The anthropometric measures; weight, crown heel length and the occipito-frontal circumference were taken. The gestational age was estimated using the modified Ballard scoring system [31]. Detailed physical examination including neurological examination, assessment of primitive reflexes, and tone were done. The level of consciousness, cry pattern and muscle tone were assessed by the researcher and computed on to the original BIND score chart [6] and each neonate was categorised as normal (BIND score 0); or into any of the 3 distinct clinical phases of ABE [6] namely subtle (BIND score 1-3), moderate (BIND score 4-6) and advanced (BIND score 7-9).
A micro-capillary sample for estimation of total serum bilirubin was taken and the total serum bilirubin was measured with the point of care serum bilirubin meter (neobil plus® machine) at presentation to assess if patients met the inclusion criteria. Laboratory estimation of serum bilirubin was done to confirm bilirubin level; and serum albumin, bicarbonate and random blood sugar were collected from each neonate to aid in appropriate subject selection because these can act as confounders. All those neonates with abnormal test results were excluded.
Healthy age and sex matched neonates presenting for BCG vaccination at the immunisation clinic were enrolled as controls. History and detailed physical/neurological examination including anthropometry was done for the controls. However, no blood samples were taken from them.
All enrolled neonates were subjected to BAER audiometry within two hours of admission [32] using Senterio® advanced device (Serial number: 300232, calibration date: 20/06/2015). During the procedure, each neonate was placed in a position of maximum comfort on the mother’s laps or an examination couch, after being fed to avoid agitation and excessive muscle activity which may cause artefacts. The electrodes were colour-coded: Electrodes were applied thus; red was placed on the mastoid, black on the cheek and white on the forehead. Electro-acoustic transducers (headphones) were held against the ear of the neonate (the red transducer on the right ear and the blue on the left), taking care that external auditory canal did not collapse. A stimulus of 10 clicks/second, rate of 30Hz, tone bursts of 2-1-2 cycles and impedance of 6kΩs at 35dB, 50dB and 75dB was used to elicit the BAER waves. Automated BAER screeners displayed the results of the test as either ‘pass’ or ‘fail’. Auditory impairment was defined as a bilateral ‘fail’ while bilateral ‘pass’ or unilateral ‘pass’ was considered normal [33]. Absolute latencies of wave I, III, and V at 75dB, 50dB and 35dB were noted. The degree of hearing loss as measured in decibels was considered as mild (failed BEAR at 35dB), moderate (failed BEAR at 50dB), severe (failed BEAR at 75dB). The test took between 15-30 minutes with optimal testing conditions.
The neonates were managed according to the unit’s protocol based on the recommendations of the American Academy of Paediatrics [11]. All neonates identified to have BAER abnormalities were referred to the audio vestibular physician for further diagnostic evaluation and treatment, in addition to being followed-up at the neonatology clinic.
Statistical Analysis
The data was entered into Statistical Package for Social Sciences (SPSS) version 21 for analysis. The data was summarised and presented as quantitative and qualitative variables and were depicted using tables and charts. Quantitative variables were expressed as mean and Standard Deviation (SD), and were compared by student’s t-test. Qualitative variables were expressed as frequencies and percentages; and Chi-square test or fisher’s-exact (where applicable) was used to establish association between the qualitative variables. The sensitivity, specificity, positive predictive value, negative predictive value and diagnostic accuracy of BIND score relative to BAER were calculated. ROC curve analysis was done to determine the accuracy of the sensitivity and specificity of the BIND score relative to the BAER as a predictor of ABE. The level of statistical significance was set at p≤0.05.
Results
General Characteristics of Neonates with Hyperbilirubinaemia and Healthy Matched Controls
A total of 66 neonates with Total Serum Bilirubin (TSB) ≥10.0 mg/dL as cases and 66 healthy term neonates as controls were enrolled, making a total of 132 age and sex matched study population. Using the students’ t-test, the mean gestational age at birth of cases was 39.1±0.8 weeks, which was comparable to that of healthy controls at 39.1±0.7 weeks (p=0.906). The age range of the neonates on presentation was between 24 hours to 15 days, with a median of 5 days. Most neonates with hyperbilirubinaemia {28 (42.4%)} and healthy neonates {26 (39.4%)} presented between day 4 and 5 after birth. Jaundice was noticed between the second and third day in 40 (60.6%) of the neonates with significant hyperbilirubinaemia.
Thirty-six (54.5%) males and 30 (45.5%) females with hyperbilirubinaemia were enrolled (M:F=1.2:1). There were 35 (53%) females and 31 (47%) males amongst the control group (M:F=1:1.1). There was no significant statistical difference between the genders of the two groups (χ2=0.758, p=0.486).
Anthropometric Indices
Using the students’ t-test, the differences in mean weight, length, occipito-frontal circumference, ponderal index, estimated gestational age between the two groups is shown in [Table/Fig-1].
Anthropometric indices of the neonates with hyperbilirubinaemia and healthy controls.
| Anthropometry | Cases | Controls | p-value |
|---|
| Mean±SD | Mean±SD |
|---|
| Weight (g) | 3060.6±568.4 | 3118.8±465.4 | 0.525 |
| Length (cm) | 47.2±2.6 | 46.9±2.3 | 0.470 |
| OFC (cm) | 35.9±1.6 | 35.1±1.1 | 0.625 |
| PI | 2.8±0.5 | 2.9±0.5 | 0.108 |
| EGA (weeks) | 39.1±0.8 | 39.1±0.7 | 0.906 |
OFC: Occipito-frontal circumference; PI: Ponderal index; EGA-Estimated gestational age; SD:Standard deviation
Abnormal BAER and BIND Score amongst Neonates with Hyperbilirubinaemia and Healthy Neonates
Of the 132 neonates studied, 20 of 66 (30.3%) cases were observed to have ABE, while no control was detected as having ABE using the BIND score. While 16 of 66 (24.2%) cases had abnormal BAER result signifying the presence of ABE. Only 2 (3%) healthy neonates had abnormal BAER findings.
Sensitivity, Specificity, Positive Predictive Value, Negative Predictive Value and Diagnostic Accuracy of BIND Score Relative to BEAR
[Table/Fig-2] shows the distribution of cases based on their BIND score and BAER findings. Using the contingency table [Table/Fig-3], the sensitivity and specificity of the BIND score relative to the BAER were 93.8% and 90% respectively. The positive predictive value was 75%, the negative predictive value was 97.8% and an overall diagnostic accuracy of 90.9%.
Relationship between BIND score and BEAR among cases.
| BIND score | BEAR | Total n (%) | χ2 | p-value |
|---|
| Normal n (%) | Abnormal n (%) |
|---|
| Normal (0) | 45 (68.2) | 1 (1.5) | 46 (69.7) | 49.08 | <0.001 |
| Subtle (1-3) | 4 (6.1) | 1 (1.5) | 5 (7.6) |
| Moderate (4-6) | 1 (1.5) | 4 (6.1) | 5 (7.6) |
| Advanced (7-9) | 0 (0) | 10 (15.1) | 10 (15.1) |
| Total | 50 (75.8) | 16 (24.2) | 66 (100) | | |
n: Number of neonates; %: Percentage; χ2: Fishers-exact test
Contingency table of BIND score and BAER among cases.
| BIND score | BAER | Total |
|---|
| Abnormal | Normal |
|---|
| Abnormal (1-9) | TP=15 | FP=5 | 20 |
| Normal (0) | FN=1 | TN=45 | 46 |
| Total | 16 | 50 | 66 |
TP: True positive; FP: False negative; FN: False negative; TN: True negative
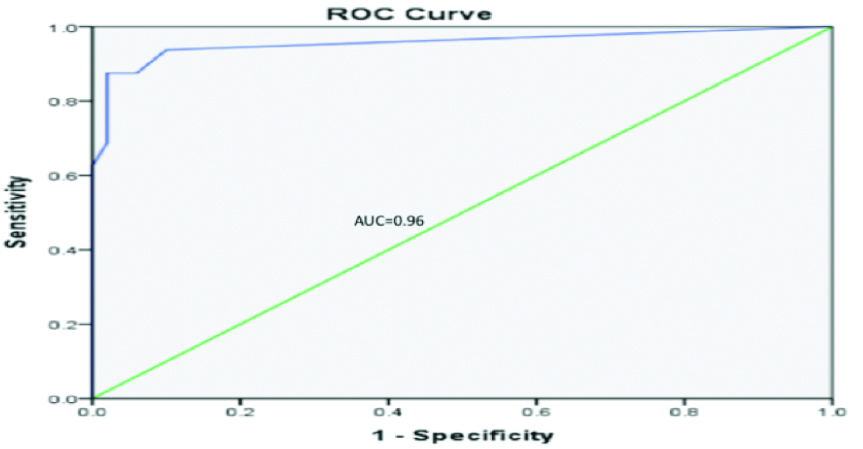
ROC curve is a plot of true positives (sensitivity) and false positives (1- specificity) across various cut-offs. The location of the curve at the upper left-hand corner indicates that the BIND score has a great discriminant capacity for neonates with ABE and those without ABE. The Area Under the Curve (AUC) is a measure of sensitivity and specificity, it defines the validity of the BIND score when compared to the BAER. [Table/Fig-4] shows an AUC of 0.96, signifying that the sensitivity and specificity of the BIND score relative to the BAER is an excellent predictor of ABE.
ROC curve evaluating sensitivity and specificity of BIND score relative to BAER as predictor of ABE.
ROC: Receiver operator characteristic; AUC: Area under the curve=0.96
Discussion
There was an 8-fold risk in the prevalence of abnormal BAER in neonates with hyperbilirubinaemia (24.2%) than in healthy term neonates (3%) in this study. In the auditory system, the area most sensitive to bilirubin toxicity is the brainstem auditory nuclei [11]. This is the most common and earliest manifestation of ABE, [2,9,14,15] thus explaining why the prevalence of abnormal BAER (which assesses the auditory pathway) is higher amongst neonates with significant hyperbilirubinaemia than healthy neonates. Furthermore, we found the diagnosis of ABE using the BIND score to have higher prevalence (30.3%) when compared with 24.2% using BAER on the neonates with hyperbilirubinaemia. This may be because the parameters assessed for by the BIND score are done subjectively based on intuition, with the features of subtle encephalopathy which are mostly non-specific more difficult to assess; while the BAER audiometry is automated, with a sensitivity of 100%, [7] as such superior to diagnosis made on clinical grounds. Out of the 5 neonates with non-specific symptoms adjudged to have subtle encephalopathy using the BIND score, only one was confirmed to have abnormal BAER. There is thus the likelihood of over estimating ABE using the BIND score when it comes to identifying non-specific subtle signs of encephalopathy. This is supported by reports of low sensitivity of the M-BIND score in identifying subtle encephalopathy (positive predictive value of 61.2%), however, it was found to be more specific in identifying moderate and advanced encephalopathy (88.9%) [5].
The present study shows the sensitivity of the BIND score relative to BAER in identifying neonates with ABE to be high (93.8%). This implies that the BIND score correctly identified more than 9 out of 10 true positive cases of ABE that were identified using BAER. The high sensitivity of the BIND score makes it very useful when it is negative, and therefore, it will help in ruling out ABE. For neonates with ABE, this high sensitivity will ensure that less than 1 in 10 will likely be undetected, thus preventing under-diagnosis of this preventable and permanent sequel. The present study also found that BIND score has a specificity of 90% relative to the BAER, implying that it will correctly detect 9 out of 10 neonates without ABE as normal, although one (10%) may be incorrectly identified as having the complication (false positive). The disadvantage of this is that, the neonates that are incorrectly classified as having ABE will be subjected to aggressive treatment with intensive phototherapy and Exchange Blood Transfusion (EBT) unnecessarily. Arguably, with the consequence of missing a case of ABE being permanent brain damage (kernicterus) or death, it is reasonable and safer to over treat than under treat, thus making the use of BIND score ideal. Furthermore, using the ROC analysis, the diagnostic ability of the BIND score to correctly classify those with and without ABE was found to be excellent with an AUC of 0.96. The value approaching one (1) indicates that the BIND score relative to the BAER is an excellent predictor of ABE. The sensitivity and specificity however, have limited clinical usefulness as they cannot be used to estimate accurately the probability of a disease condition [33]. The Positive Predictive Value (PPV) and the Negative Predictive Value (NPV) of BIND score relative to the BAER (which are better measures of predicting ABE) were 75% and 97.8% respectively. This implies that the BIND score when compared with BAER has 75% degree of accuracy in identifying ABE and 97.8% certainty of ABE being absent if the score is 0 (normal). The relatively low PPV, as reported previously [5] may be linked with the low precision of the score in clinical diagnosis of subtle ABE (as the features are non-specific), [6] similar to the findings in this study (out of 5 neonates with subtle ABE using the BIND score, only 1 had abnormal BAER). In clinical practice, the high NPV is important because out of every 100 neonates adjudged to be normal, only 2 will be falsely reported as having ABE and subjected to aggressive treatment, while the remaining 98 will be correctly identified as normal. The diagnostic accuracy of the BIND score relative to BAER of 90.9% implies that the score will correctly categorise neonates as normal or having ABE 9 out of 10 times when compared with BAER. The BIND score is thus capable of improving the detection of neonates with ABE for appropriate timely interventions, as well as reducing the rate of unnecessary EBT in neonates without ABE. However, both PPV and NPV vary with changing prevalence of a disease [33]. It will thus not be applicable to populations whose prevalence of ABE is significantly different from the 24.2% prevalence reported in this study. If the PPV were applied to a population with very low prevalence, the number of false-positive results will be far higher than the number of true-positive results, leading to labelling of normal neonates as abnormal and subsequent unnecessary treatment. The NPV of the test will also change in a similar but reverse direction to PPV.
In a similar study [5] the sensitivity of the modified BIND (M-BIND) score was reported to be 90.7% with a PPV of 88.9%, while the specificity was 97.7% with a NPV of 98.2%. These findings were however based on the accuracy of the M-BIND score in identifying neonates with scores of ≥3. If the accuracy of the M-BIND score was however considered from cut-off score of ≥1, which was also the cut-off used in the present study, the sensitivity of the M-BIND was 98.1%, specificity was 88.2%, PPV was 61.2% and NPV was 99.6% and are thus comparable with the present study. The slight observed differences when compared with the present study may be because this study used BAER as a standard for comparison, which is superior to the basis for comparison used in the M-BIND score study (clinical acumen/experience of consultants over residents), as such the result from this study provides more reliable data on the usefulness of BIND score. The lower the M-BIND score, the less precise the diagnosis of ABE as shown by the low PPV of 61.2% at cut-off score of ≥1 (due to the non-specific features of subtle ABE which can be missed) and vice versa (PPV increased to 88.9% at cut-off score of ≥3). This is similar to what was obtained in this study. Moreover, the study that validated the M-BIND score (which incorporates evaluation of eye signs) was used, as opposed to the present study which used the original BIND score (with no eye signs), both of which are screening tests that have not been validated using the same confirmatory test, so their performance may be different.
Limitation
A potential criticism of the study is that it was conducted in a single centre which may limit generalisation of findings. However, it is believed that most patients with severe form of jaundice are referred to our facility, which is the only tertiary hospital that offers neonatal services, and thus results are representative of the population. Also, findings from this study may only be useful in developing countries with similar high prevalence of ABE and are unlikely to be applicable in the developed settings where the prevalence of ABE is extremely low compared with the study site. Another limitation is the use of BAER as the standard for diagnosing ABE. Although autopsy is the confirmed gold standard in diagnosing BE, it was not feasible in our environment due to cultural and religious factors militating against its use. BAER is a suitable alternative to detect outcome due to its high predictive values in detecting SNHL, the hallmark for ABE diagnosis. Finally, BAER was done within 2 hours of presentation. It would have been better if a repeat were done after treatment, preferably pre-discharge to provide more information on reversal of damage with appropriate treatment. The cost of the investigation was a limiting factor in a follow-up repeat test. The causes of hyperbilirubinaemia were not looked into for use in planning and prevention.
Area of Future Research
Future research should explore the relationship between BIND score, BAER and cranial magnetic resonance imaging to determine and compare their diagnostic accuracy in predicting ABE; and identify factors that predispose some neonates to developing encephalopathy while others are protected.
Conclusion
This study has demonstrated the value of BAER as a useful diagnostic tool in the care and management of the neonate with significantly raised serum bilirubin. Specifically, this study has also shown the value of clinical assessment using the BIND score as a reliable alternative where BAER is not available. The use of BIND score in diagnosing BE can be relied upon in settings where the expertise and facilities for BAER audiometry are not available.