Utility of Carba NP test (Inhouse/RAPIDEC Commercial Kit) in the Identification of Carbapenemase Producing Clinical Isolates in a Tertiary Care Hospital
K Sreeja Vamsi1, S Ramamoorthy2, TS Murali3, Manisha Singh4, Vasanti Kabra5
1 Research Scholar, Department of Microbiology, Manipal Academy of Higher Education, Manipal, Karnataka, India.
2 CEO, Department of Microbiology, Palamur Biosciences Pvt. Ltd., Mahabubnagar, Telangana, India.
3 Associate Professor, Department of Biotechnology, Manipal Academy of Higher Education, Karnataka, India.
4 Professor, Department of Microbiology, SVS Educational Society, Mahabubnagar, Telangana, India.
5 Professor and Head, Department of Microbiology, SVS Educational Society, Mahabubnagar, Telangana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. S Ramamoorthy, Palamur Biosciences Pvt. Ltd., Mahabubnagar, Telangana, India.
E-mail: ram.murthy@palamurbio.com; drsinghmanisha@gmail.com
Introduction
Carbapenems are the drugs of choice for the treatment of many multidrug resistant hospital acquired infections. Resistance to carbapenems also is not uncommon and is increasingly being reported now-a-days. Though various tests like modified hodge test are available for the detection of carbapenemases, they lack sensitivity and specificity. Recently, the Carba NP test has been introduced for carbapenemase detection which has been approved and included in CLSI guidelines.
Aim
To identify the prevalence of carbapenemase producing isolates and the utility of Carba NP in house as well as commercial RAPIDEC Carba NP test in the identification of these carbapenemases.
Materials and Methods
All the carbapenem resistant organisms isolated during the study period from July 2018 to December 2018 were included in the study. A total of 91 isolates were identified during the study period which were further tested for carbapenemase production by both in House Carba NP test as well as commercially available RAPIDEC Carba NP (Biomerieux) as per the existing protocols. Twenty five carbapenem sensitive isolates were also tested. Klebsiella pneumoniae BAA ATCC-1705 and BAA ATCC-1706 were used as controls.
Results
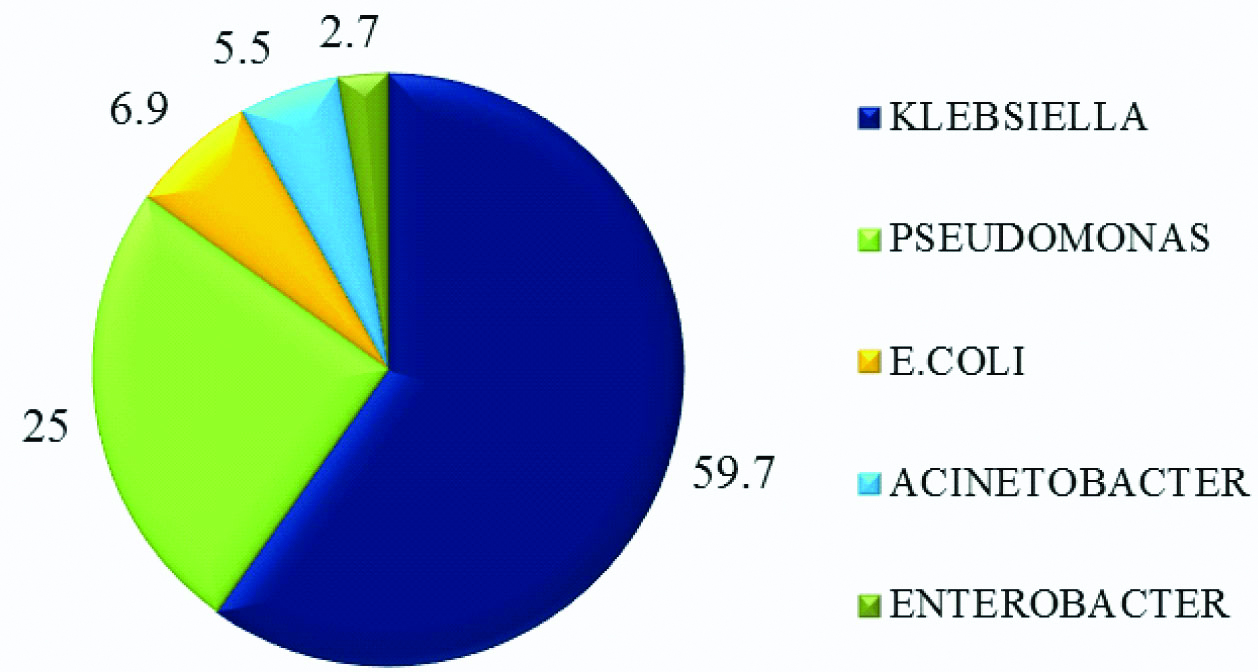
Out of 91, carbapenem resistant strains tested, 72 were identified as carbapenemase producers. Out of them, Klebsiella pneumonia accounted for 59.7% of the total carbapenemase producing organisms followed by Pseudomonas aeruginosa (25%) and E.coli (6.9%) followed by Acinetobacter and Enterobacter which constituted less than 5%. Among enterobacteriaceae, 43 out of 59 carbapenem resistant Klebsiella were carbapenemase producers whereas all the 5 carbapenem resistant E.coli were positive for Carba NP test. Among Pseudomonas 18 out of 21 were carbapenemase producers. All the positive isolates by in-house Carba NP test were also positive by commercial RAPIDEC test and vice-versa and none of the 25 carbapenem sensitive strains tested were positive for Carba NP test by either method indicating 100% correlation between the two methods studied.
Conclusion
Carba NP in house as well as commercial RAPIDEC Carba NP test were equally effective in the identification of the carbapenemase producing gram negative bacilli. Wide adaptability of these tests by various laboratories will help in the early identification of these potentially spreading carbapenemase producers, which in association with appropriate treatment and infection control practices will prevent the emergence of these strains and will decrease the mortality and morbidity.
Enterobacteriaceae, Klebsiella pneumonia, Multidrug resistance, Pseudomonas aeruginosa
Introduction
Carbapenems are the drugs of choice for the treatment of many multidrug resistant hospital acquired infections. With the increasing emergence of carbapenem resistant bacteria in the recent past, it has become extremely difficult to treat these infections as only very few alternatives exist to treat such infections. This results in increased mortality and morbidity in hospitals and also imparts great economic burden on the health care system. There is a higher likelihood for the spread of these carbapenemase producers in the hospital settings which further adds to this complication. Rapid identification of these carbapenemase producers in association with appropriate treatment and infection control practices will help in the containment of these infections [1,2].
There are various mechanisms that lead to carbapenem resistance in bacteria among which carbapenemase production is a major contributor and is widespread among the Gram negative organisms [2,3]. Other mechanisms like efflux pumps, altered function of porins or binding proteins also contribute to carbapenem resistance to some extent. Though various tests like modified hodge test are available for the detection of carbapenemases, they lack sensitivity and specificity. On the other side, molecular techniques like polymerase chain reaction have good sensitivity and specificity, yet needs expert handling and infrastructure. In this scenario, there is a need for a rapid, cheap and easily performable test for the detection of carbapenemase production in clinical strains. Recently, the Carba NP test has been introduced for carbapenemase detection which has been evaluated, approved and included in CLSI guidelines [4]. The test is based on the change in the pH using indicator phenol red (change in colour from red to yellow or orange) caused by the hydrolysis of imipenem by a bacterial lysate. The report shows Enterobacteriaceace to be 100% sensitive and specific, whereas it is 94.4% sensitive for Pseudomonas in screening carbapenemases [5,6]. CLSI also states that the rapid carbapenemase detection methods are having more than 90% of sensitivity and specificity with regards to enterobacteriaceae members like Klebsiella pneumoniae where NDM, VIM and IMP type of resistance is more common where as their sensitivity and specificity are low for non-fermenting organisms like Acinetobacter where OXA48 carbapenemases are common mechanisms involved [4].
Many researchers have identified the importance of rapid carbapenemase detection methods both in India and abroad and the sensitivity and specificity varied between different studies. Overall the sensitivity of RAPIDEC carba NP tests was between 90% to 100% and specificity varied from 96% to 100% [7-10]. Though many authors in India have evaluated these rapid carbapenemase detection methods in their clinical set up. Prevalence of carbapenemase producing organisms in our Telangana state have not been studied well by using these rapid methods like RAPIDEC carba NP tests. As the prevalence of these carbapenemase producers varies from place to place, it is important that they should be identified promptly for both epidemiological and therapeutic purposes. Thus, the present study aims to identify the prevalence of carbapenemase producing isolates and compare the utility of an in-house Carba NP test to the commercial RAPIDEC Carba NP test in the detection of these carbapenemase producing isolates in and around Mahabubnagar district, Telangana, India.
Materials and Methods
This was a prospective study done in the Department of Microbiology, SVS Medical College from July 2018-December 2018. Ethical clearance certificate (SVSMC/IEC Approval/No.05/2018-623) was taken from the institutional ethical committee. All gram negative bacterial isolates from patients admitted in the hospital were included and all the gram positive isolates and samples isolates from outpatient were excluded from the study. A total of 612 Gram negative organisms were isolated during the study period. Clinical samples such as pus, blood, sputum, stool, urine, endotracheal aspirates and other body fluids were taken-up for the study. Samples were identified up to the species level and antibiotic sensitivity testing was done by Vitek 2 systems as per standard protocols which has usual sensitivity of 99% and specificity of 96% [11]. All the carbapenem resistant organisms isolated were further tested for carbapenemase production. The carbapenemase production was tested using both commercially available RAPIDEC Carba NP test (Biomerieux) as per the standard protocols and an in-house Carba NP test.
For the in-house carba NP test, two 1.5 mL Eppendorf tubes were taken for each strain and labeled as A and B. 200 μL of sterile water was added to tube A and emulsified with 2-3 loop full of bacterial colony to make a heavy suspension. From this, 100 μL of the inoculum was transferred to tube B. Separately, Solution A (0.05% phenol red, 10 mM ZnSO4, pH 7.8) and Solution B (1 mL sol A, 6 mg imipenem powder) were prepared. Now, 100 μL of sol A was added to tube marked A and 100 μL of sol B was added to tube B and both the tubes were incubated at 37°C for 2 hours. A change in colour from red to yellow was considered as positive Carba NP test indicating carbapenemase production [10].
Twenty five carbapenem sensitive isolates were also included for the analysis. The positive and negative controls used were Klebsiella pneumoniae ATCC BAA-1705 and Klebsiella pneumonia ATCC BAA-1706.
Procedure Flow chart
Culture of the clinical samples → identification of the organism → detection of carbapenem resistance → detection of carbapenemase production by RAPIDEC carba NP + in-house Carba NP test → result obtained in 2 hours.
Statistical Analysis
Statistical analysis was performed by SPSS VERSION 20 and graph pad prism software version 6.0. The collected data was entered in to MS excel and tabulated. Sensitivity, specificity, positive predictive and negative predictive values were detected.
Results
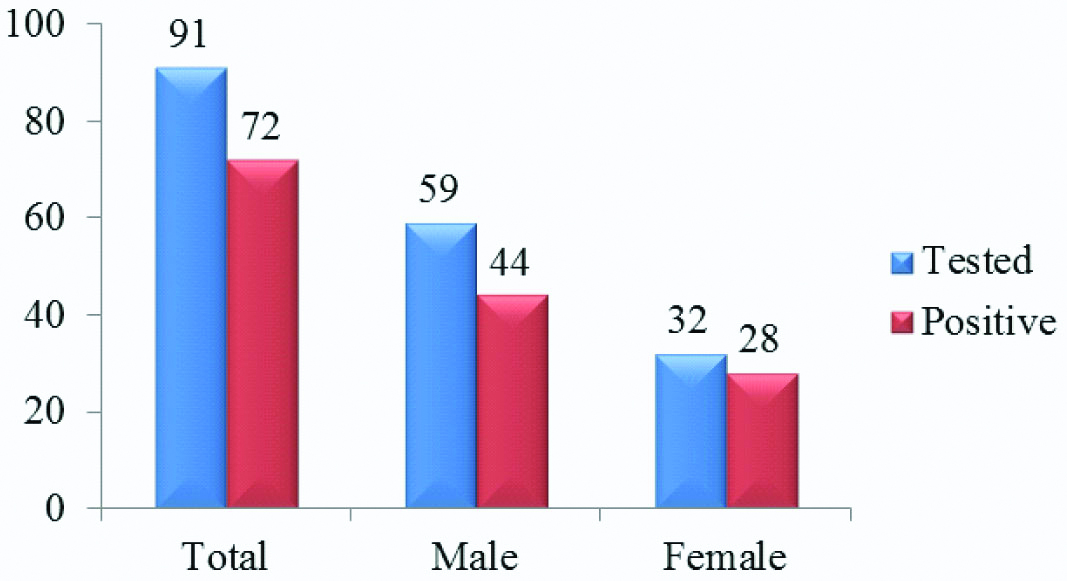
A total 612 Gram negative organisms were isolated during the study period, out of which 91 isolates were identified as carbapenem resistant and were further tested for carbapenemase production by both our in-house Carba NP test as well as RAPIDEC Carba NP test [Table/Fig-1] Out of the 91 carbapenem resistant strains tested, 72 were identified as carbapenemase producers in both the methods. Out of them, Klebsiella pneumoniae accounted for 59.7% of the total carbapenemase producing organisms followed by Pseudomonas aeruginosa (25%) and Escherichia coli (6.9%). Acinetobacter and Enterobacter constituted less than 5% of the strains showing carbapenemase production [Table/Fig-2]. Among Enterobacteriaceae, 43 out of 59 carbapenem resistant Klebsiella were carbapenemase producers whereas all the 5 carbapenem resistant E.coli were positive for Carba Np test. Among Pseudomonas, 18 out of 21 isolates were carbapenemase producers. The performance of the test in detecting carbapenemase production was high when considering the enterobacteriaceae members like E.coli and Klebsiella where as it was low for non-fermenters like Acinetobacter. Out of 72 carbapenemase producing organisms, 44 positive cases are from males and 28 are from females as shown in [Table/Fig-3].
Results from both in house Carba NP test (left side) and RAPIDEC carba NP (Biomerieux) (right side) showing positive for carbapenemase production.

Distribution of carbapenemase producing bacterial isolates in the present study.
Gender wise distribution of the isolates obtained and those that tested positive for carbapenemase production in the present study.
All the positive isolates identified by our in-house Carba NP test were also positive by commercial RAPIDEC test and none of the 25 carbapenem sensitive strains tested were positive for carbapenemase production by either method indicating 100% correlation between the two methods studied.
The overall sensitivity of the test was 79.12% (CI:69.33% to 86.94%) and specificity was 82.79% (CI: 71.48% to 85.14%). The positive predictive value PPV was 82.91% (CI:71.48% to 85.14%). and negative predictive value NPV was 79.38% (CI:71.65% to 94.08%).
[Table/Fig-4] shows the distribution of carbapenemase positive Gram negative bacteria from various clinical samples. Klebsiella pneumoniae was the predominant carbapenemase producer and most of the carbapenemase producing GNB were isolated from endotracheal secretions, followed by blood and others.
Distribution of carbapenemase positive Gram negative bacteria from clinical samples (n=72).
| Bacterial genus | Sputum and BAL | Blood | Pus | Urine | ET | Stool | Total |
|---|
| Klebsiella | 7 | 9 | 6 | 3 | 18 | 0 | 43 |
| Pseudomonas | 2 | 8 | 4 | 0 | 4 | 0 | 18 |
| E. coli | 1 | 0 | 0 | 0 | 2 | 2 | 5 |
| Acinetobacter | 0 | 1 | 0 | 1 | 2 | 0 | 4 |
| Enterobacter | 0 | 1 | 0 | 0 | 1 | 0 | 2 |
| Total organisms | 10 | 19 | 10 | 4 | 27 | 2 | 72 |
Discussion
Multidrug resistance is increasingly being observed both in community acquired and hospital acquired pathogens. Carbapenems are the drugs of choice in majority of the serious nosocomial infections. With the wide spread emergence of carbapenem resistance especially in enterobacteriaceae members like Klebsiella, it has become extremely difficult for the clinicians to treat such cases as only few alternatives like tigecycline and colistin are available for the treatment [12]. As the antibiotic pipeline is almost empty, it is mandatory that the emergence of resistance to carbapenems is contained by early identification and adherence to appropriate infection control measures along with the best clinical practices. Sound knowledge about the mechanisms of carbapenem resistance and identification methods will help in achieving this goal. As carbapenemase production is the predominant mechanism for resistance to carbapenems, early detection of carbapenemase producing organisms is extremely important in preventing the spread of these infections.
Nordmann P et al., developed a novel method for rapid detection of these carbapenemase producers [5]. The basic principle of this test was the hydrolysis of carbapenem by the bacterial enzyme carbapenemase which detects the pH changes by measuring the change of the colour of phenol red indicator. Based on this principle, many commercial kits have been developed among which RAPIDEC Carba NP test developed by Biomerieux is widely used with good sensitivity and specificity [10,13,14]. Our in-house Carba NP test was also based on the same principle.
In the present study, both in-house Carba NP and commercial RAPIDEC Carba NP tests were evaluated in their ability to detect carbapenemase producing isolates. Out of the total 91 carbapenem resistant isolates tested, 72 were identified to be positive for carbapenemase production by both the tests. In addition, none of the carbapenem sensitive isolates tested were positive by either test. Klebsiella was the most common isolate showing carbapenemase production. Gupta V et al., tested 75 bacterial strains and found all strains to be positive for carbapenemase production by both Carba NP and RAPIDEC Carba NP tests [10]. They also observed Klebsiella as the most common bacterial isolate showing carbapenemase production in their study. But they have also used other methodologies like Modified Carba NP (MCNP) test, Carbapenem Inactivation Method (CIM) test. Similarly, Dortet L et al.. compared RAPIDEC Carba NP, the Rapid CARB Screen and the Carba NP test and found that sensitivity and specificity were 99% and 100%, respectively for the RAPIDEC Carba NP test, 96.8% and 100% for the Carba NP test [15]. They have stated that RAPIDEC Carba NP possesses the best performance for rapid and efficient detection of carbapenemase-producing Enterobacteriaceae and suggested it as a first-line screen of carbapenemase-producing Enterobacteriaceae in clinical settings. Comparatively, limited literature is available in India against western countries with regards to the utilisation of rapid carbapenemase detection methods [16-18]. Wide utilisation of these rapid detection methods in a country like India will help not only in decreasing the mortality and morbidity but also the health care economical burden.
RAPIDEC carba NP test was extremely useful in the early detection of carbapenemase producing isolates in our hospital because of the rapid result which can be obtained within 2 hours and also doesn’t needed any technical expertise. Because of this early detection, appropriate infection control practices could be placed which further decreased the spread of these infections. The high cost of RAPIDEC carba NP test may limit its use routinely in clinical laboratories in low settings but the inhouse Carba NP test is a viable option for its routine use in such settings as it is quite cheap and easy to perform and interpret.
Though various methodologies are available for the detection of carbapenemases, the methods should be individualised in accordance with the needs, work load and economical constraints of the hospitals. Nevertheless, rapid detection of these infections plays a major role in the appropriate management of the patients and initiation of infection control measures thus preventing the spread of these infections.
Limitation
The limitation of this study, is it has less sensitivity towards OXA-48 carbapenemases. No genotyping of these isolates was done to identify any resistance mechanisms underlying.
Conclusion
Both the Carba NP in-house as well as commercial RAPIDEC Carba NP test were equally effective in the identification of the carbapenemase producers among the Gram negative bacilli. Wide adaptability of these tests by various laboratories will help in the early identification of these potentially spreading carbapenemase producers, which in association with appropriate treatment and infection control practices will prevent the emergence and decreases the mortality and morbidity associated with these bacterial infections.
Declaration: Author had presented the Abstract (Oral Presentation) in International Conference held at Manipal, Karnataka, India.
Author Declaration:
Financial or Other Competing Interests: Yes (As declared above)
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Sep 14, 2019
Manual Googling: Oct 21, 2019
iThenticate Software: Nov 28, 2019 (6%)
[1]. Garg A, Garg J, Swaroop A, Kala S, Rao YK, Kumar A, Emerging carbapenemases: Bringing a step closer to extremely drug resistant bacteriaInt Int J Curr Res 2011 3:35-38. [Google Scholar]
[2]. Bush K, Fisher JF, Epidemiological expansion, structural studies, and clinical challenges of new b-lactamases from gram-negative bacteriaAnnu Rev Microbiol 2011 65:455-78.10.1146/annurev-micro-090110-10291121740228 [Google Scholar] [CrossRef] [PubMed]
[3]. Nordmann P, Dortet L, Poirel L, Carbapenem resistance in Enterobacteriaceae: here is the storm!Trends Mol Med 2012 18:263-72.10.1016/j.molmed.2012.03.00322480775 [Google Scholar] [CrossRef] [PubMed]
[4]. CLSI. “Performance standards for antimicrobial susceptibility testing,” Twentieth informational supplement, Clinical and Laboratory Standards Institute Doc. M100ED28-2018 [Google Scholar]
[5]. Nordmann P, Poirel L, Dortet L, Rapid detection of carbapenemase producing EnterobacteriaceaeEmerg Infect Dis 2012 18:1503-07.10.3201/eid1809.12035522932472 [Google Scholar] [CrossRef] [PubMed]
[6]. Dortet L, Poirel L, Nordmann P, Rapid detection of carbapenemase-producing Pseudomonas sppJ Clin Microbiol 2012 50:3773-76.10.1128/JCM.01597-1222972829 [Google Scholar] [CrossRef] [PubMed]
[7]. Ho PL, Wang Y, Wing-Sze Tse C, Fung KS, Cheng VC, Lee R, Rapid detection of carbapenemase production in enterobacteriaceae by use of a modified paper strip Carba NP methodJ Clin Microbiol 2018 56(1):pii: e01110-17.10.1128/JCM.01110-1729070653 [Google Scholar] [CrossRef] [PubMed]
[8]. Vanstone G, Woodhead S, Roulston K, Sharma H, Wey E, Smith ER, Improving the detection of carbapenemase-producing organisms (CPO) in a low-prevalence setting: Evaluation of four commercial methods and implementation of an algorithm of testingJournal of Medical Microbiology 2018 67:208-14.10.1099/jmm.0.00067429388538 [Google Scholar] [CrossRef] [PubMed]
[9]. Garg A, Garg J, Upadhyay GC, Agarwal A, Bhattacharjee A, Evaluation of the Rapidec Carba NP test kit for detection of carbapenemase- producing Gram-negative bacteriaAntimicrob Agents Chemother 2015 59:7870-72.10.1128/AAC.01783-1526416868 [Google Scholar] [CrossRef] [PubMed]
[10]. Gupta V, Soni R, Jain N, Chander J, In vitro cost-effective methods to detect carbapenemases in EnterobacteriaceaeJ Lab Physicians 2018 10:101-05.10.4103/JLP.JLP_25_1729403215 [Google Scholar] [CrossRef] [PubMed]
[11]. Nonhoff C, Rottiers S, Struelens MJ, Evaluation of the Vitek 2 system for identification and antimicrobial susceptibility testing of Staphylococcus sppClin Microbiol Infect 2005 11:150-53.10.1111/j.1469-0691.2004.01047.x15679491 [Google Scholar] [CrossRef] [PubMed]
[12]. Nordmann P, Cuzon G, Nass T, The real threat of Klebsiella pneumonia carbapenemase -producing bacteriaLancet Infect Dis 2009 9:228-36.10.1016/S1473-3099(09)70054-4 [Google Scholar] [CrossRef]
[13]. Mancini S, Kieffer N, Poirel L, Nordmann P, Evaluation of the Rapidec Carba NP and beta-Carba tests for rapid detection of carbapenemase-producing EnterobacteriaceaeDiagn Microbiol Infect Dis 2017 88:293-97.10.1016/j.diagmicrobio.2017.05.006 [Google Scholar] [CrossRef]
[14]. Poirel L, Nordmann P, Rapidec Carba NP test for rapid detection of carbapenemase producersJ Clin Microbiol 2015 53:3003-08.10.1128/JCM.00977-1526085619 [Google Scholar] [CrossRef] [PubMed]
[15]. Dortet L, Poriel L, Nordmanne P, Evaluation of the RAPIDEC CARBA NP, the Rapid CARB Screen and the Carba NP test for biochemical detection of carbapenemase-producing EnterobacteriaceaeJ Antimicrob Chemother 2015 70:3014-22.10.1093/jac/dkv21326260131 [Google Scholar] [CrossRef] [PubMed]
[16]. Tejasvi K, Anuradha B, Detection of carbapenamase production by rapid carba NP test among Enterobacteriaceae isolates in tertiary care hospitalIndian J Microbiol Res 2019 6(3):272-76.10.18231/j.ijmr.2019.059 [Google Scholar] [CrossRef]
[17]. Rao MR, Chandrasekhar P, detection of carbapenemase production in enterobacteriaceae and Pseudomonas species by carbapenemase Nordmann-Poirel testJ Lab Physicians 2019 11:107-10.10.4103/JLP.JLP_132_1831160847 [Google Scholar] [CrossRef] [PubMed]
[18]. Mangayarkarasi V, Moses SP, Swarna SR, Kalaiselvi K, Fathima S, I In-House standardisation of Carba NP test for carbapenemase detection in gram negative bacteriaInt J Curr Microbiol App Sci 2018 7(01):2876-81.10.20546/ijcmas.2018.701.342 [Google Scholar] [CrossRef]