Haemoglobinopathies include thalassaemias and other structural variants of haemoglobin, having an autosomal recessive pattern of inheritance. It affects nearly 3 to 4 lac of newborns every year, mostly affecting the lower socioeconomic countries [1]. The structural variants of thalassaemia occur as a result of substitution of one or more amino acids in the globin chain of the haemoglobin molecule [2]. The interaction between thalassaemia and its structural variants pose a foremost health hazard among the Indian population [3]. Children suffering from thalassaemia major remain transfusion dependant and are thus affected by severe disease related problems. Hence, it is essential to correctly identify the carriers among the expected mothers by using a sensitive screening method to reduce the disease burden [4]. Previously, used battery of tests for detection of haemoglobinopathies were time consuming, less accurate and required more manpower. Recently, CE-HPLC is functional for quantification of normal and abnormal haemoglobins which is clinically relevant and ideal for screening purpose due to its high sensitivity and less inter-observer variability [5]. The main aim of this study was identification of the carrier stages of haemoglobinopathies by using a sensitive screening method (CE-HPLC) and to evaluate the epidemiology of thalassaemia as a part of routine screening amongst antenatal mothers, and to maximally identify the heterozygotes for further prevention of the disease.
Materials and Methods
This single centered, prospective, cross-sectional study was conducted amongst the antenatal mothers visiting the Thalassaemia Control Unit (TCU) of the Department of Pathology, Institute of Post Graduate Medical Education and Research, Kolkata, West Bengal, India. Proper approval was obtained from the ethical committee of the institution and informed consent was taken individually from the patients (IEC no- IPGME&R/IEC/2018/569). The study population was 7340 and study period was from January 2015 to December 2017, for a duration of three years. Antenatal mothers visiting the TCU of our department were included within the study. No stringent exclusion criterion was applied but the mothers who were unwilling to undergo the test and those suffering from severe anaemia and who underwent recent blood transfusions were excluded from the study. Due to various social reasons and low literacy rate, majority of antenatal mother’s husband attending the antenatal clinic did not give consent for sample collection. The data was collected from the database which was maintained and organised by the Linux Based Thalamon Software (Venus IT Solutions). For blood sampling and HPLC procedure, the samples were run in the Bio-Rad Variant II Haemoglobin Testing System. (Hercules, California, USA).
The variant β-thalassaemia short program pack was used. It is comprised of two elution buffers namely-elution buffer 1 and elution buffer 2, both composed of sodium phosphate. The other components included a whole blood primer consisting of lyophilized human red blood cell haemolysate with preservatives, a haemolysis reagent and a wash solution having deionized water within it. An HbA2/F calibrator/diluent set consisting of lyophilized human red blood cell haemolysate along with deionized water was also used. Besides, sample vials, cation exchange analytical cartridges ROM (Read Only Memory) card and CD-ROM were there. The fully automated machine, Bio-Rad Variant II machine in which the procedure was done, determines the values of haemoglobin A2 and F and also helps in qualitative detection of abnormal haemoglobin.
1-2 mL of blood samples were collected from antenatal mothers in EDTA vials and kept at 2-8°C. For the procedure, 1.0 mL of haemolysis reagent and 5 μL of anticoagulated whole blood were added in each sample and were analysed in batches. The procedure was concluded within a week. At an interval of 6.5 minutes, the samples were simultaneously injected. Further analysis of the samples was done on a cation exchange stream. This process was done using a phosphate ion gradient which was generated by mixing two buffers of different ionic strengths for washing out the different types of haemoglobin. At the beginning of each cycle, HbA /F calibrator and two level controls were analysed.
A dual wavelength filter photometer analysed the haemoglobin, washing out the solvent from the cartridge by detecting the absorbance of light changes at 415 nm whereas the secondary filter at 690 nm adjusted the baseline for effects caused by mixing buffers with different ionic strengths.
Statistical Analysis
IBM SPSS statistics software, version 19 and MedCalc software, version 12.3.0.0 was used for performing the statistical analysis. The mean±Standard Deviation (SD) was determined for analysis of the continuous variables whereas categorical variables were represented as frequencies and percentages.
Results
A total of 7340 of antenatal mothers were screened for β thalassaemia traits along with other haemoglobinopathies. [Table/Fig-1] shows the trend analysis of normal and affected individuals among the study population. On HPLC study, out of the total subject’s majority were diagnosed as normal. Amongst the carrier stages, majority was diagnosed as β thalassaemia carriers, followed by HbE carrier and rest were diagnosed among others as HbD carriers, HbS carrier and HPFH traits. The mean age of the antenatal mothers screened for the study duration was 24.75±4.70 years for normal individuals. The other variables showed similar results [Table/Fig-2].
Trend analysis of normal and haemoglobinopathies among the study population.
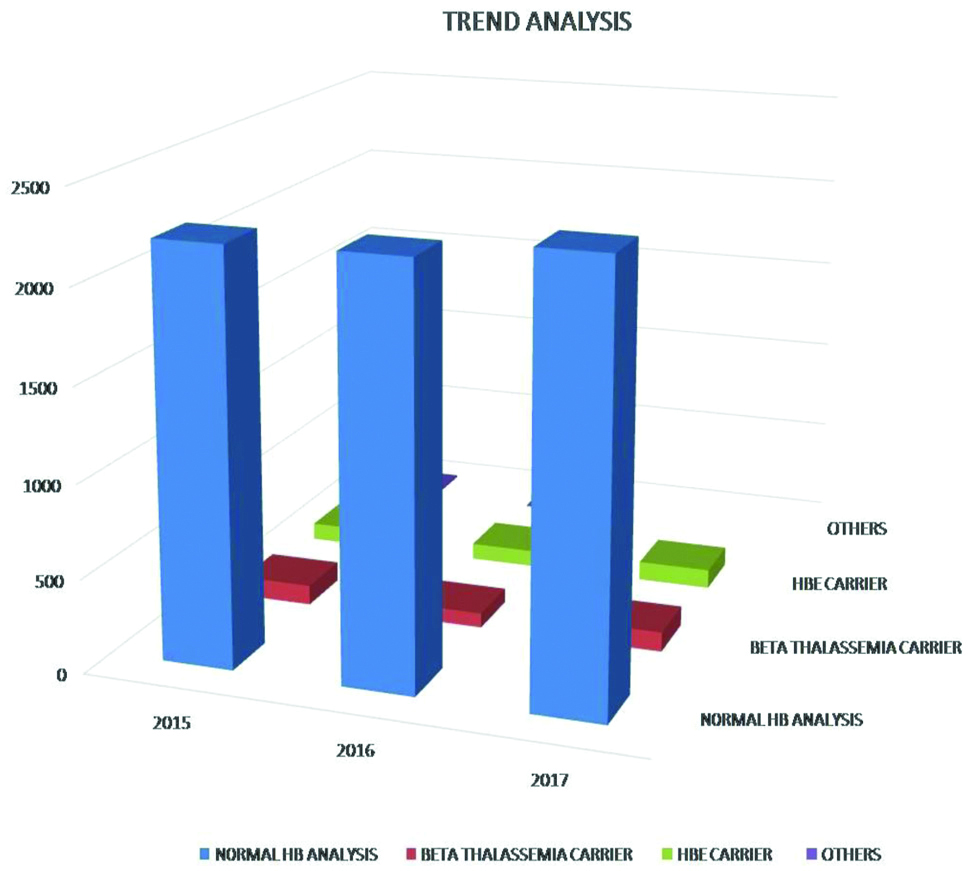
Distribution of study population.
| Parameters | Normal haemoglobin analysis | β thalassaemia carrier | HbE carrier | Others |
|---|
| Year of study (N=7340) |
| 2015 | 2206 | 106 | 93 | 12 |
| 2016 | 2219 | 78 | 88 | 6 |
| 2017 | 2313 | 105 | 102 | 10 |
| Total | 6738 | 291 | 283 | 28 |
| Age (p-value is 0.4436, considered not significant) |
| Mean±SD | 24.75±4.70 | 24.55±4.56 | 24.28±4.45 | 24.44±4.21 |
| Minimum | 14 | 16 | 17 | 17 |
| Maximum | 42 | 38 | 40 | 34 |
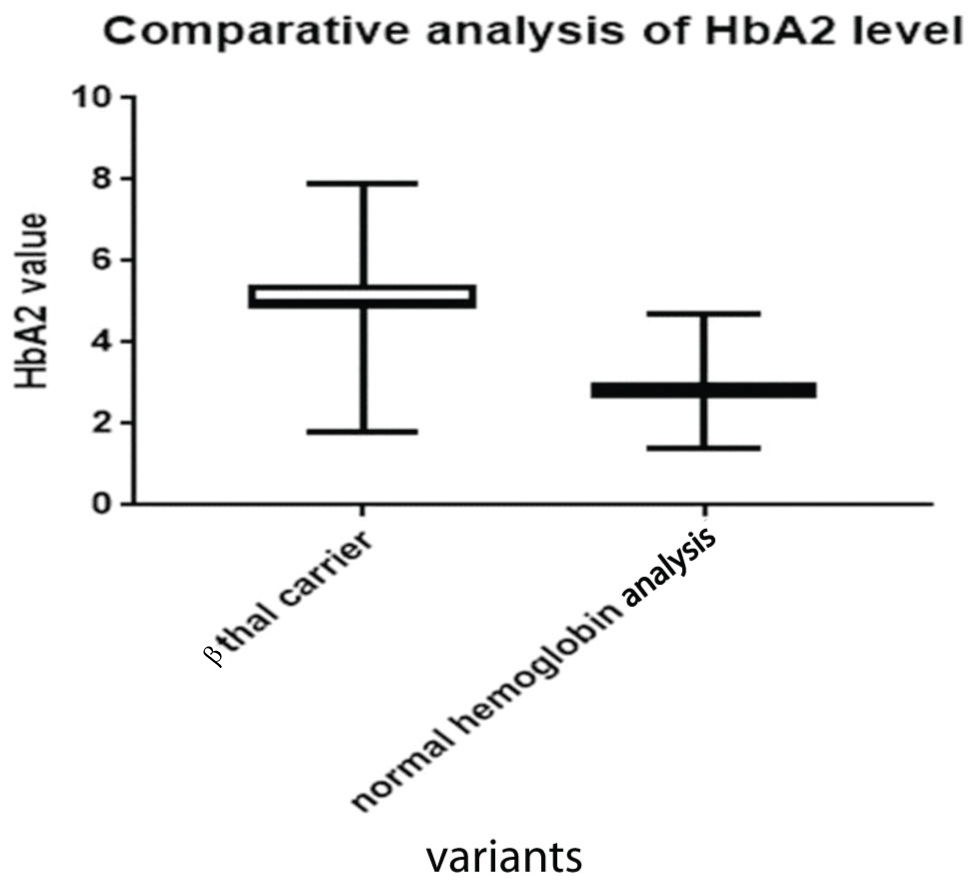
HbA2 levels were assessed among the normal population and those detected with β thalassaemia carriers among the study group [Table/Fig-3]. Group A were designated as those having normal haemoglobin analysis and were compared with Group B, who were designated as those detected with β thalassaemia carrier. Group A individuals were found to have a significantly lower value with respect to β thalassaemia carrier, thus helping in proper categorisation and further follow-up. The median value for Group A was lower than Group B. The Box- Whisker plot represents and correlates with the aforesaid comparison [Table/Fig-4].
Analysis of HbA2 levels in β thalassaemia carriers and its comparision with normal subjects.
| Group A | Group B |
|---|
| Title | Normal Hb analysis | β Thalassaemia carrier |
|---|
| Mean (g/dL) | 2.78 | 5.074 |
| Standard deviation | 0.27 | 0.52 |
| Sample Size (N) | 6738 | 291 |
| Standard error of mean | 0.0032 | 0.030 |
| Lower 95% of confidence interval | 2.78 | 5.01 |
| Upper 95% of confidence interval | 2.79 | 5.13 |
| Minimum | 1.40 | 1.80 |
| Median (50th percentile) | 2.80 | 5.0 |
| Maximum | 4.70 | 7.9 |
| Normality test Ks | 0.094 | 0.091 |
| Normality test p-value | <0.0001 | <0.0001 |
| Passed normality test? | No | No |
Comparative analysis of HbA2.
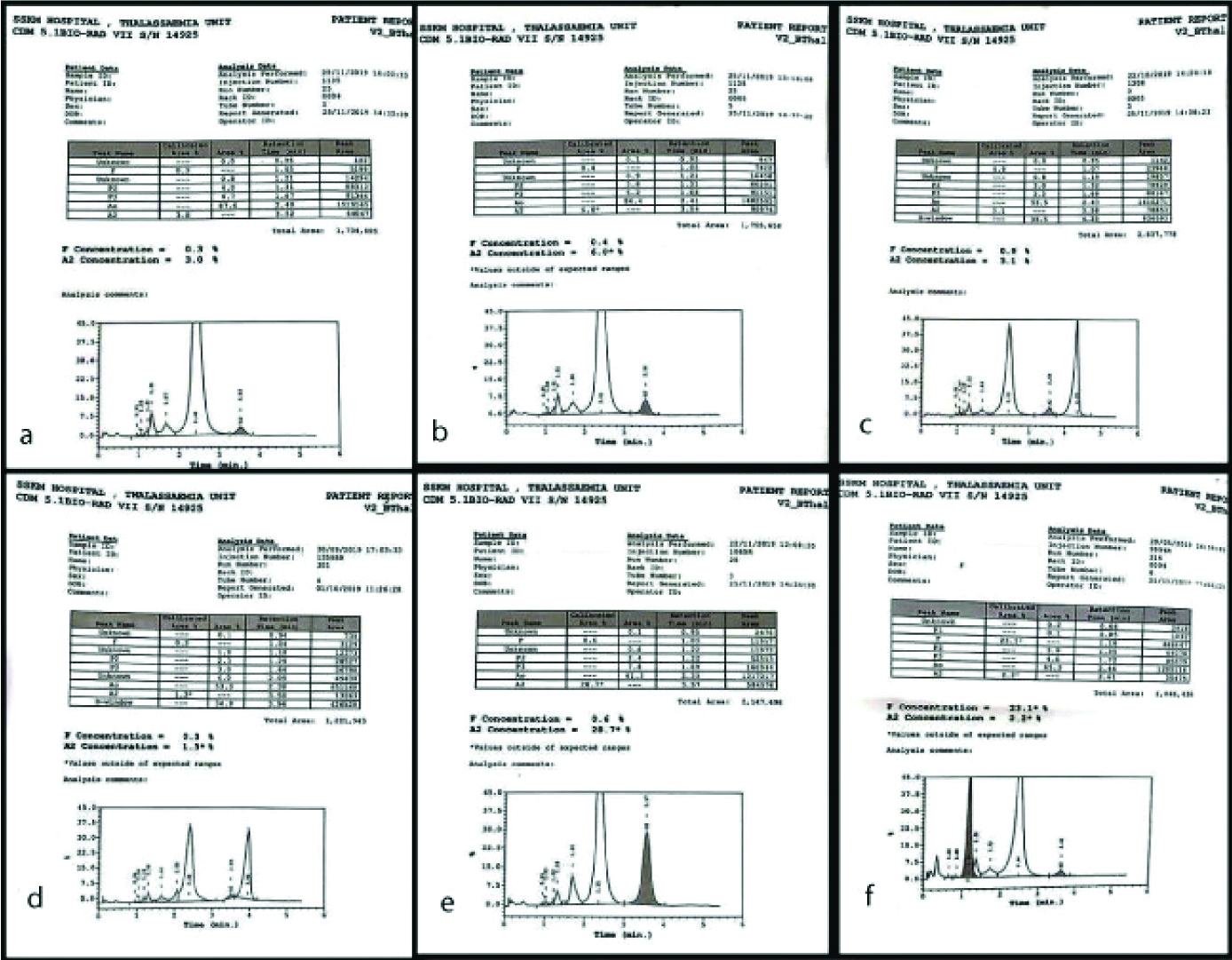
The different haematological parameters (Hb level, haematocrit, MCV, MCH, and MCHC) were assessed within the study population [Table/Fig-5]. All the parameters in Hb E carriers were seen to decrease than the normal individuals but less significantly decreased than β thalassaemia traits. For HbD carriers, as compared to the normal individuals there was a decrease in the haematological parameters, except the mean levels MCHC which was seen to be increased with respect to the normal individuals. The parameters were seen to decrease than normal individuals for Hb S carriers, but not significantly whereas there was still an increase in the MCHC levels, almost similar to HbD. In mothers who had HPFH, there was a decrease in the mean levels of MCV and MCH parameters, with almost same levels of the other parameters to that of normal individuals. [Table/Fig-6] shows CE-HPLC of normal, and other thalassaemia carriers.
Assesment of haematological parameters in different groups of carriers and normal subjects
| Diagnosis | Number | Statistical Analysis | Hb Level (g/dL) | HCT (%) | MCV (fl) | MCH (pg) | MCHC (g/dL) |
|---|
| Normal Hb analysis | 6738 | Mean±SD | 11.41±3.02 | 34.05±4.18 | 85.13±11.99 | 28.69±5.5 | 32.91±1.72 |
| Lower 95% CI | 11.31 | 33.79 | 84.74 | 28.51 | 32.85 |
| Upper 95% C1 | 11.8 | 34.31 | 85.51 | 28.87 | 32.96 |
| β Thalassaemia carrier | 291 | Mean±SD | 9.84±1.00 | 31.83±3.28 | 60.33±20.00 | 20.94±1.87 | 30.87±0.91 |
| Lower 95% CI | 10.76 | 31.28 | 66.38 | 20.62 | 30.71 |
| Upper 95% C1 | 11.10 | 32.39 | 73.10 | 21.26 | 31.02 |
| HB E carrier | 283 | Mean±SD | 10.93±1.02 | 33.35±3.15 | 69.74±22.17 | 25.59±1.83 | 32.78±0.96 |
| Lower 95% CI | 9.67 | 32.2 | 57.18 | 25.29 | 32.63 |
| Upper 95% C1 | 10.01 | 33.86 | 73.48 | 25.88 | 32.94 |
| HB D carrier | 7 | Mean±SD | 10.93±1.06 | 32.73±3.27 | 79.76±9.37 | 26.66±3.35 | 33.4±0.30 |
| Lower 95% CI | 8.2 | 24.60 | 56.48 | 18.34 | 32.65 |
| Upper 95% C1 | 13.5 | 40.86 | 103.5 | 34.99 | 34.14 |
| HB S carrier | 18 | Mean±SD | 11.44±1.04 | 30.52±2.20 | 82.4±5.1 | 27.85±2.83 | 33.7±1.64 |
| Lower 95% CI | 10.77 | 29.12 | 79.2 | 26.05 | 32.65 |
| Upper 95% C1 | 12.10 | 31.92 | 85.79 | 29.64 | 34.74 |
| HPFH trait | 3 | Mean±SD | 11.43±0.83 | 34.7±2.47 | 78.76±9.37 | 25.93±1.77 | 32.86±0.23 |
| Lower 95% CI | 9.3 | 28.60 | 64.72 | 21.51 | 32.29 |
| Upper 95% C1 | 13.5 | 40.92 | 92.81 | 30.35 | 33.44 |
a) CE-HPLC chromatogram of normal; b) CEHPLC chromatogram of β thalassaemia trait; c) CE-HPLC chromatogram of HbS carrier; d) CE-HPLC chromatogram of HbD carrier; e) CE-HPLC chromatogram of HbE carrier; f) CE-HPLC chromatogram of HPFH.
Discussion
Haemoglobinopathies are the inherited disorders which include both qualitative and quantitative abnormalities of haemoglobin. Approximately, 5-7% of the world population carries an abnormal Haemoglobin gene [6]. Due to low socio-economic status, lack of awareness and uneven access to standard medical services, it is difficult to implement one standard screening protocol in India. So, we have selected the most vulnerable group the antenatal mother attending the antenatal clinic of our hospital for screening because at that time expected mothers are concerned about the newborns’ well being and agreed to follow necessary action. Screening of couples at first antenatal visit in the first trimester is the standard recommendation [7]. In present study only 9.2% of the study population was sampled during their first trimester which cautioned to take necessary action to prevent birth of a diseased child. Choudhuri S et al., also documented similar finding [8].
CE-HPLC is an emerging tool taking an upper hand over the electrophoresis in the last decade, due to its sensitivity, reliability and precision in identification and quantification of normal Hb and its abnormal variants. Thus, the use of this method is appropriate for the screening purpose [9]. A limitation of this technique stands as α thalassaemia, A2 β thalassaemia and other haemoglobinopathies that co-elute in the same frequencies in HPLC are difficult to distinguish, but this can be overcome by proper clinical correlation and expertisation in this field [10].
Due to the higher prevalence of β thalassaemia traits in our country, it necessitates the utmost need for antenatal screening and further prevention of thalassaemia major among the off springs [11].
The detection of other structural variants of abnormal haemoglobin (Hb E, HbS, HbD, HPFH) is imperative to prevent extensive bleeding due to their interactions in homozygous and double heterozygous cases. The prevalence of haemoglobinopathies varies widely in different demographic areas of our country. HbE carrier is maximum in North Eastern and Eastern India, ranging from 3-50%. We found 3.8% of HbE carriers in present study which was higher as compared to the 2.6% of cases reported in northern India by Sachdev R et al., [10]. HbS is prevalent among the different districts of Maharashtra and Gujarat ranging from 5-35% [12-14]. In this study from eastern India, we found 0.2% of HbS carriers which is lower than reported aforementioned percentage. Owing to these regional variations of haemoglobinopathies in our country, extensive sub population study is required to identify the zonal discrepancy and emphasise screening in the high risk areas. West Bengal possess a high thalassaemic burden with much less awareness specially amongst the rural population. Therefore, the screening programs in antenatal period should be voluntary by increasing mass awareness. Consecutive follow-up is essential especially among the carriers. Optimum detection of heterozygotes at the incipient stage of the disease with an easily accessible method is extremely important.
In the multicentric Jai Vigyan Programme Indian Council of the Medical Research on Community Control of Thalassaemia, the awareness about thalassaemia among pregnant women in the six cities were found out to be very limited varying from 0.2-4.8% in Bangalore, Mumbai, Ludhiana, Vadodra, Dibrugarh and around 20.7% in Kolkata. The report of this programme also highlighted a lack of awareness, knowledge and practice regarding thalasssemia among the rural population [11]. So, increased awareness and mass screening including both rural and urban population from different regions is very essential in present scenario. Different states are currently undergoing different thalassaemia control programmes and screening is being done on specific target groups. There are two nodal centres with 21 centres under them in different districts in the West Bengal State, Thalassaemia Control Programme. There is varied regional prevalence of β thalassaemia ranging from 4-10%. For screening on a large database, to create awareness and counseling in varied regions of the state, a software known as Thalman has been developed [15].
In present study, 7340 antenatal mothers for three consecutive years, the distribution of study population was assessed [Table/Fig-2]. The cut-off of HbA2 was taken as 3.5% for β thalassaemia carriers. On trend analysis, it was seen the data for the successive two years corresponds with the preceding years and also with the total study population [Table/Fig-1]. The HbA2 levels were also analysed and compared between normal subjects of the study population and β thalassaemia carriers [Table/Fig-3]. The median HbA2 levels in normal subjects were 2.78 g/dL and that of carriers was 5.074 g/dL. The comparative analysis is shown in the Box Whisker plot [Table/Fig-4]. The results were corroborating with other reported studies [16]. The different haematological parameters (Hb level, haematocrit, MCV, MCH, and MCHC) were analysed and compared among the normal study population and different carrier groups [Table/Fig-5]. Hence, it was seen that among the parameters, the widest variation was shown by the MCV levels. The haematological parameters of Hb D showed greatest variations with respect to the lower and upper confidence intervals. β thalassaemia carrier showed the most significant decrease in the mean levels of all the parameters as compared to the normal individuals of the study population. So, evaluation of different haematological parameters helps in solving the cases of co-elution of different haemoglobin variants. But, considering the low socio-economic status, iron deficiency anaemia should be ruled out by other ancillary investigations and different discrimination indices [17].
During the first antenatal visit, mothers with reduced RBC indices should be screened for HbA2 and HbF, so as to maximally identify the number of heterozygotes. A national control programme with cumulative effort from tertiary care centre, thalassaemia societies, NGOs are highly appreciated to create a mass awareness about antenatal screening and control of major thalassaemia and related disorders [18].
In our previous work, we have reported the spectrum of haemoglobinopathies in our region among all respondent categories [19]. Now, we are reporting the spectrum of haemoglobinopathies in antenatal mothers.
Limitation
Co-elution of A2 β thalassaemia and other haemoglobinopathies was a limitation of the technique itself. Due to various social reasons and low literacy rate majority of antenatal mother’s husband attending the antenatal clinic did not give consent for the analysis which hindered the screening and awareness process. A lack of routine access to molecular testing was another drawback.
Conclusion
The present study revealed that CE-HPLC serves as an appropriate tool for routine screening of thalassaemia. It helps in detection of the carrier stages at its earliest and helps in prevention of the disease. β thalassaemia traits and HbE carriers being the most prevalent haemoglobinopathies in our region, it necessitates screening of antenatal mothers for detection of the abnormal haemoglobin carriers at an incipient stage, since the homozygous and double heterozygous interactions of these carrier stages leads to relentless bleeding. An effort towards education and awareness in a large scale is to be augmented for detection of carrier states in early pregnancy period and thereafter implementation of proper follow-up in order to reduce the immense disease burden of haemoglobinopathies.