Peri-implant diseases are a group of inflammatory diseases that mainly affects the hard and soft tissues around the dental implants which are characterised by bleeding, redness and swelling of the gingiva along with deterioration of bone [1,2]. Peri-implant Mucositis (p-iM) is a prevalent, reversible condition which is associated with redness, swelling and bleeding of the gingival tissue around the implant without radiographic evidence of bone loss [3], and if left untreated, may lead to the development of Peri-Implantitis (PI), exhibiting loss of the supporting alveolar bone [4,5].
The gold standard treatment for peri-implant diseases includes professional plaque control by non-surgical Mechanical Debridement (MD) and oral home care that includes tooth brushing and use of chemical rinses [6]. Other adjunctive measures such as provision of local and systemic antibiotics, air abrasive devices and photodynamic therapy have also been proposed as new methods for greater results [7,8]. For definitive and proper management against the occurrence of disease, the consistent removal of the regular dental biofilm is of the primary concern that can be achieved with oral hygiene exercises, along with professional interventions. However, the mentioned treatment modalities, are not essentially helpful in treating peri-implantitis. Numerous surgical methods and decontamination procedures have been introduced for the treatment of peri-implantitis, but a consensus still has not been established regarding the optimal treatment protocol and there is less scientific evidence to prove which method is superior to the other [9].
With the development in the field of periodontics, new entities are being introduced which are playing an important role in the discovery of new and innovative methods of increasing plaque control and in detection of immune modulating factors. Recent studies have reported the use of probiotics which may effectively inhibit gingival inflammation, leading to reduction in peri-implant disease [10,11]. These mediators are ‘living bacteria when dispensed in suitable quantities in the host to offer health benefit’. Probiotics are known to exacerbate the commensal flora and prevents the settlement of the pathogenic bacteria which are associated with causation of the disease. The effect of probiotic involves different mechanisms including competitive exclusion of the harmful pathogens, immune modulation and impeding their adhesion to the substrate [12].
Materials and Methods
Study Protocol Registration
The present review was written in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [13] and registered in the PROSPERO website (http://www.crd.york.ac.uk/PROSPERO) database. The record was available online on November 12, 2018, registered as CRD42018110121.
PICOS Eligibility Criteria
Placebo-controlled, blinded, RCTs were included. Publication in language other than English were excluded.
Studies including ≥10 patients per group clinically diagnosed with peri-implant diseases (p-iM and/or PI) without any age restrictions.
Clinical efficacy of probiotics in any form with active intervention, placebo, or no treatment. Studies were included when they (i) tested one or more probiotics as an adjunct to Mechanical Debridement (MD) alone or with a placebo group and (ii) tested one or more probiotics in groups categorised according to health. Some of the included probiotics were, but not limited to Lactobacillus rhamnossus, Lactobacillus curvatus, Bifidobacterium animalis, Lactobacillus brevis, Lactobacillus reuteri, and Lactobacillus plantarum.
Probing Depth (PD) was the primary outcome. Secondary outcome measures included Bleeding on Probing (BOP) and/or Plaque Index (Pi). In the present review, only trials that reported any of these outcomes with a minimum follow-up of 4 weeks were included.
Electronic Database and Study Search
Following databases were explored; MEDLINE (1952-January 2019), EMBASE (1984-January 2019), Cochrane Central Register of Controlled Trials and Cochrane Oral Health Group Trials Register (1993-January 2019) for articles that focused the PICOS question using the following terms: ((Probiotics) OR (Lactobacillus reuteri) OR (Lactobacillus rhamnossus) OR (Lactobacillus curvatus) OR (Bifidobacterium animalis) OR (Lactobacillus brevis) OR (Lactobacillus plantarum) OR (yogurt) OR (tablets) OR (lozenges) AND ((peri-implant diseases) OR (peri-implantitis) OR (peri-implant mucositis) OR (inflammation) AND (plaque) OR (plaque scores) OR (plaque index) OR (bleeding) OR (bleeding on probing) OR (probing depth).
Titles of the main articles and their abstracts were screened. If primary variable was reported in the main abstract (or complete abstract missing), the article was selected for complete-text reviewing. If the complete-text article fulfilled the selection criteria, they were subsequently included in the review. Bibliography from research studies were manually hand searched to recognise studies that had gone unnoticed during the database searching in the following scientific journals: Clinical Oral Investigations, Journal of Periodontology, Acta Odontologica Scandinavica, Journal of Periodontal Research, Journal of Prosthodontic Research and Journal of Clinical Periodontology. Relevant studies that met the inclusion criteria were later used for data abstraction. The review process was planned and design in accordance with the PRISMA guideline [13].
Data Items and Abstractions
Data abstraction was performed according to several general and clinical characteristics including study design, demographics, potential confounders, probiotic administration, follow-up period, final study results and gingival inflammatory parameters.
Risk of Bias Across and within Individual Studies
Recommendations of the Consolidated Standards of Reporting Trials statement was used for assessing the risk of bias across the studies [14]. Risk of bias for individual study was estimated using the Cochrane Handbook for Systematic Reviews of Interventions [15]. Three scoring system including ‘high’, ‘low’ or ‘unclear’ were recorded for sections that had ‘high risk of bias’, ‘low risk of bias’ or ‘unclear risk of bias’, respectively. Overall, the studies were considered ‘high quality’ if all conditions met, ‘low quality’ if ≥1 condition did not meet, or ‘moderate quality’ if ≥1 condition was partly met.
Summary Measures and Approach to Quantitative Analysis
Meta-analysis was performed considering studies that reported the outcomes using L. reuteri only. Heterogeneity was estimated using the I2 and χ2 statistics. For considerable heterogeneity crossing over 50%, the random effects model was employed, or else the fixed effects model was used for heterogeneity ≤50% [16]. Heterogeneity was considered significant if p-value presented ≤0.05. Forest plots were plotted for Weighted Mean Difference (WMD) and 95% Confidence Intervals (CI) of main results. Data that could not be used for meta-analysis were described narratively.
Grading the ‘Body of Evidence’
Grading was performed for the included studies using the ‘Grading of Recommendations Assessment, Development and Evaluation’ (GRADE) [17]. The sections that were rated included biases, reliability of the results, integrity of evidence, accuracy and extent of the effect [18].
Results
Search Results
A total of 2381 study titles and abstracts were initially identified. After removal of the duplicates (n=148), initial screening of titles and abstracts was performed, and 2221 articles were excluded that did not correspond to the PICO question. A total of 12 papers were selected for full-text reading. Of these 12 articles, six studies [10,11,19-22] were included and subsequently used for data extraction. The other six studies were excluded and not considered for this systematic review [Appendix-A]. All clinical trials were conducted in university hospitals. [Table/Fig-1] illustrates the study identification flow chart according to PRISMA with the reasons for exclusion of articles.
PRISMA flow diagram for studies retrieved through the searching and selection process.
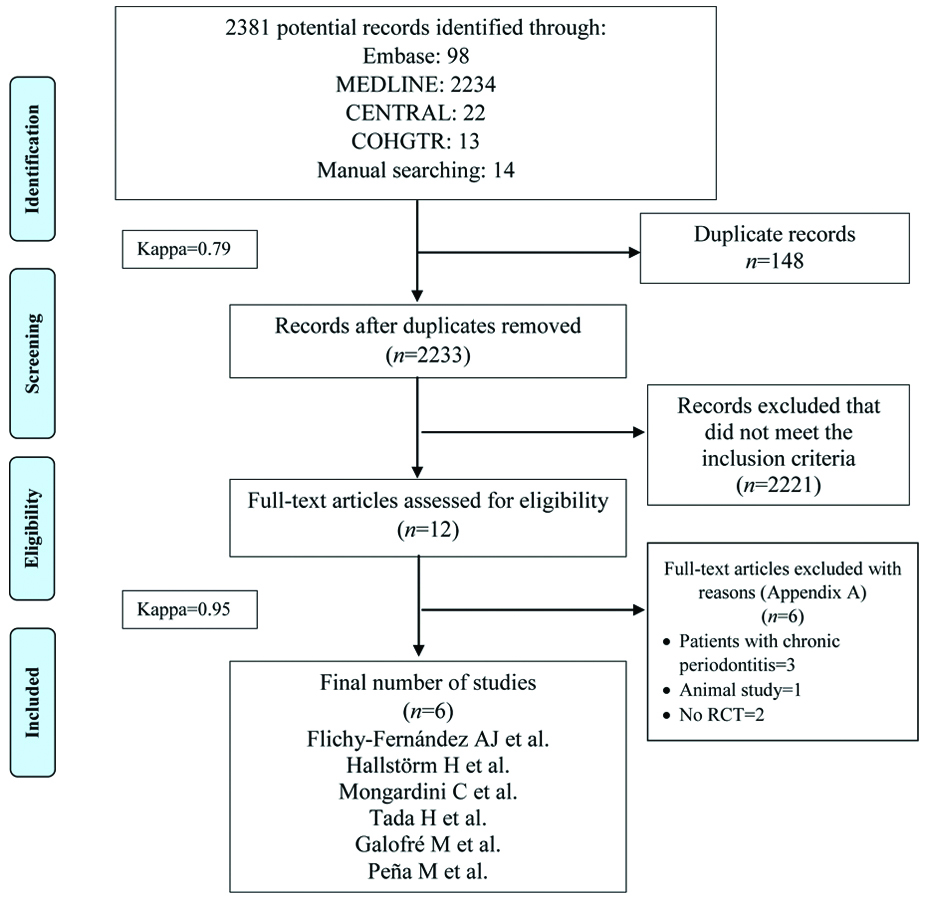
General Description of Included Studies
All published studies were either double-blind [10,11,19,20] or triple-blind [21,22] placebo-controlled RCTs. Three studies were performed in Spain [10,21,22], while remaining three studies each were performed in Sweden [11], Italy [19], and Japan [20] respectively. The mean age of the included patients ranged from 53.7 years to 68.8 years. A total of 247 dental implants were examined for therapeutic effect. Probiotics were administered to 141 individuals, while a total of 106 individuals were administered placebo. Data on the overall percentage of female subjects ranged between 27% and 80%. Four studies included smokers [11,19,20,22], out of which one study evaluated the effects of probiotics in previously treated periodontitis cases and included photodynamic therapy [19]. Among the forms of probiotics used, five studies used lozenges for L. reuteri of dosage 1×108 Colony Forming Unit (CFU) and one study used the combination L. plantarum and L. brevis as powder form. Five studies [10,11,19,21,22] evaluated the treatment outcomes of probiotics in patients with p-iM, while two studies included patients having PI [20,21]. The follow-up duration ranged from 4 to 26 weeks from non-brushing period in the included studies. Two studies were funded by university while one study was funded by private laboratory [Table/Fig-2].
General characteristics of included studies in reversed chronological order.
| Investigator et al. | Study design; Country | Sample size; Mean age in years; Female %; Number of dental implants | Confounders | Type of probiotic administration; Frequency; Dosage | Disease type; Clinical inflammatory parameters studied | Follow-up (weeks) | Study outcomes | Funding source |
|---|
| Flichy-Fernández A et al., [10] | Double-blind placebo controlled RCT; Spain | 12; 60.2; 58%; 54 | NA | Lozenges- Lactobacillus reuteri ATCC PTA 5289 + DSM 17938; x1; 1×108 CFU | p-iM: PD <4 mm with no evidence of bone lossPi, PD | Up to 4 | Significant improvement in clinical inflammatory parameters for probiotic group at follow-up | Not stated |
| Hallstörm H et al., [11] | Double-blind placebo controlled RCT; Sweden | Probiotic25; 53.7; 71%; 25Placebo24; 63.3; 56%; 24 | Smokers | Lozenges- Lactobacillus reuteri DSM 17938 + ATCC PTA 5289; x2; 1×108 CFU | p-iM: PD ≥4 mm combined with bleeding and/or pus on probingBOP, Pi, PD | Up to 26 | No statistical significant differences between test and control groups at follow-up | University funded |
| Mongardini C et al., [19] | RCT cross over; Italy | 20; 57.0; 55%; 20 | Smokers; PDT; treated periodontitis cases | Powder- Lactobacillus plantarum + Lactobacillus brevis; x1; NA | p-iM: bone loss <2 mmPi, BOP | Up to 6 | No statistical significant differences between test and control groups at follow-up | Private funded |
| Tada H et al., [20] | Double-blind placebo controlled RCT; Japan | Probiotic15; 68.80; 80%; 15Placebo15; 65.87; 67%; 15 | Smokers; antibiotics | Lozenges- Lactobacillus reuteri DSM 17938 + ATCC PTA 5289; x2; 1×108 CFU | PI: PD >4 and <7 mm with BOP and/or pus discharge and bone loss of >2 mmPD, BOP | Up to 24 | No statistical significant differences between test and control groups at follow-up | Not stated |
| Galofré M et al., [21] | Triple-blind parallel-design RCT; Spain | Group- p-iMProbiotic11; 61.5; 45%; 11Placebo11; 60.0; 27%; 11Group- PIProbiotic11; 61.7; 64%; 11Placebo11; 56.8; 55%; 11 | NA | Lozenges- Lactobacillus reuteri DSM 17938 + ATCC PTA 5289; x1; 1×108 CFU | p-iM: inflamed mucosa with BOP and/or suppuration and no evidence of bone lossPI: BOP and/or suppuration, PD ≥5 mm and bone loss of ≥2 mmPi, BOP, PD | Up to 13 | No statistical significant differences between test and control groups of p-iM at follow-upNo statistical significant differences between test and control groups of PI at follow-up | Not stated |
| Peña M et al., [22] | Triple-blind parallel-design RCT; Spain | Probiotic25; 55.96; 68%; 25Placebo25; 61.16; 48%; 25 | Smokers | Lozenges- Lactobacillus reuteri (DSM 17938 + ATCC PTA 5289; x1; NA | p-iM: presence of bleeding with gingival redness, swelling, and BOP without bone loss PD, Pi, BOP | Up to 18 | No statistical significant differences between test and control groups at follow-up | University funded |
BOP: Bleeding on probing; CFU: Colony forming unit; NA: Not available; PD: Probing depth; PDT: Photodynamic therapy; Pi: Plaque index; p-iM: Peri-implant mucositis; PI: Peri-implantitis; RCT: Randomised clinical trial
Clinical Peri-implant Parameters of the included Studies
Peri-implant mucositis
A total of 5 clinical trials reported data on PD [10,11,20-22] which ranged from 2.46 mm to 3.7 mm in the probiotics group and 2.47 mm to 3.5 mm for placebo groups at follow-up, respectively. Four RCTs [11,19,21,22] reported data on BOP out of which two trials [11,22] reported data as mean percentages and one study [19] reported BOP as median and interquartile ranges. Mean percentage of BOP ranged from 14% to 64% in the probiotics group, while mean percentage of BOP ranged from 17% to 60% in the placebo groups at follow-up, respectively. Five studies [10,11,19,21,22] reported data on Pi out of which two trials reported data as mean percentages and one trial reported data as median and interquartile ranges. Overall mean Pi of probiotics and placebo groups ranged from 0.25 to 0.96 and 0.29 to 1.09, while mean percentage Pi of probiotics and placebo groups ranged from 12% to 24% and 15% to 28% at follow-up, respectively [Table/Fig-3].
Clinical peri-implant outcomes of the included studies in chronological order.
| Investigators et al., Year | Pocket depth (mm) | Bleeding on probing | Plaque index |
|---|
| Flichy-Fernández A et al., [10] | ProbioticBaseline: 3.55±0.40Follow-up: 2.46±0.92* | NA | ProbioticBaseline: 1.77±1.20Follow-up: 0.96±1.15* |
| PlaceboBaseline: 2.47±0.79Follow-up: 2.65±0.84 | PlaceboBaseline: 1.09±1.12Follow-up: 1.09±1.12 |
| Hallstörm H et al., [11] | ProbioticBaseline: 4.3±1.1Follow-up: 3.7±1.3* | Probiotic†Baseline: 54Follow-up: 14* | Probiotic†Baseline: 26Follow-up: 12* |
| PlaceboBaseline: 4.0±1.4Follow-up: 3.5±1.5 | Placebo†Baseline: 58Follow-up: 17* | Placebo†Baseline: 32Follow-up: 15* |
| Mongardini C et al., [19] | NA | Probiotic‡Baseline: 4 (3-6)Follow-up: 2 (0-2)* | Probiotic‡Baseline: 1.2 (0.92-1.59)Follow-up: 0 (0.00-0.17)* |
| Placebo‡Baseline: 3.5 (2-4)Follow-up: 2 (0-3)* | Placebo‡Baseline: 1.42 (0.92-1.75)Follow-up: 0.17 (0.00-0.33)* |
| Tada H et al., [20] | ProbioticBaseline: 3.90±0.46Follow-up: 3.21±0.84* | ProbioticBaseline: 3.20±1.26Follow-up: 1.53±1.41* | ProbioticBaseline: 1.67±0.72Follow-up: 1.13±0.74* |
| PlaceboBaseline: 4.04±1.14Follow-up: 3.47±0.95* | PlaceboBaseline: 3.67±1.59Follow-up: 2.33±1.95* | PlaceboBaseline: 1.47±0.74Follow-up: 1.20±0.68 |
| Galofré M et al., [21] | Group- p-iMProbioticBaseline: 3.84±0.55Follow-up: 3.35±0.76* | Group- p-iMProbioticBaseline: 0.61±0.27Follow-up: 0.29±0.09* | Group- p-iMProbioticBaseline: 0.41±0.21Follow-up: 0.25±0.10* |
| PlaceboBaseline: 3.82±0.64Follow-up: 3.66±0.62 | PlaceboBaseline: 0.42±0.18Follow-up: 0.35±0.22 | PlaceboBaseline: 0.39±0.10Follow-up: 0.29±0.10* |
| Group- PIProbioticBaseline: 5.07±0.87Follow-up: 4.53±0.72* | Group- PIProbioticBaseline: 0.53±0.23Follow-up: 0.33±0.09* | Group- PIProbioticBaseline: 0.44±0.14Follow-up: 0.28±0.24* |
| PlaceboBaseline: 4.90±0.66Follow-up: 4.70±0.75 | PlaceboBaseline: 0.49±0.23Follow-up: 0.39±0.17 | PlaceboBaseline: 0.43±0.21Follow-up: 0.33±0.28* |
| Peña M et al., [22] | ProbioticBaseline: 3.10±0.74Follow-up: 2.88±0.62* | Probiotic†Baseline: 100Follow-up: 64* | Probiotic†Baseline: 72Follow-up: 24* |
| PlaceboBaseline: 3.32±0.65Follow-up: 2.98±0.60* | Placebo†Baseline: 100Follow-up: 60* | Placebo†Baseline: 68Follow-up: 28* |
*Indicates intra-group statistical significance, p-iM: Peri-implant mucositis; PI: Peri-implantitis, †Values reported in percentage, ‡Values reported in median and interquartile range
Peri-implantitis
For peri-implantitis, a total of 2 clinical trials [20,21] reported PD which ranged from 3.2 mm to 4.5 mm in the probiotics group and 3.47 mm to 4.7 mm in the placebo group at follow-up, respectively. Data for BOP ranged from 0.33 to 1.53 in the probiotic group and 0.39 to 2.33 in the placebo group at follow-up. Plaque index was also reported in the same RCTs which ranged from 0.28 to 1.13 in the probiotic group and 0.33 to 1.2 in the placebo group at follow-up, respectively [Table/Fig-3].
Risk of Bias Assessment Across Studies
All the clinical studies were subjected to critical analysis following the Cochrane Handbook for Systematic Reviews of Interventions for evaluating the risk of bias. The present authors classified four clinical trials [19-22] as having a low risk of bias and two clinical trials [10,11] as having a high risk of bias. These four studies were judged to have lower risk of bias due to adequate reporting of randomisation technique, sequence generation, blinding and patients withdrawal. In contrast the domain classified as having high risk of bias was sequence generation, allocation concealment, and unclear reporting of randomisation methods in two clinical trials [Table/Fig-4].
Risk of bias of the included studies.
| Investigators | Randomization methods | Sequence generation | Allocation concealment | Blinding of study participants and personnel | All patients accounted for at the end of study | Clear explanation of withdrawals | Selective reporting | Over risk of bias |
|---|
| Flichy-Fernández A et al., [10] | Unclear | High | High | Low | Low | Low | Low | High |
| Hallstörm H et al., [11] | Low | High | Low | Low | Low | Low | Unclear | High |
| Mongardini C et al., [19] | Low | Low | Low | Low | Low | Low | Low | Low |
| Tada H et al., [20] | Low | Low | Low | Low | Low | Low | Low | Low |
| Galofré M et al., [21] | Low | Low | Low | Low | Low | Low | Low | Low |
| Peña M et al., [22] | Low | Low | Low | Low | Low | Low | Low | Low |
Main Outcome of the Studies
On intra-group analysis from the studies, all clinical studies showed that probiotic administration was effective in the treatment of peri-implant diseases at follow-up. After performing statistical meta-analysis, no statistical significant differences in the clinical peri-implant inflammatory parameters was observed between probiotics and placebo groups at follow-up for both peri-implant mucositis and peri-implantitis, respectively.
Meta-analysis was performed for quantitative data assessment. Only data presented for L. reuteri were analysed for clinical periodontal inflammatory parameters in meta-analysis.
Effect of probiotics in peri-implant mucositis
Considering the effects of probiotics on p-iM, 4 studies for PD [10,11,19,22], while only 2 studies for BOP and Pi presented data to be included in the meta-analysis [11,22]. The overall effect for PD was calculated as WMD, while Odds Ratio (OR) was calculated for both BOP and Pi, respectively, as their data was presented as numbers with positive outcomes. Fixed effect model was employed as there was no significant heterogeneity observed for PD (χ2=1.48, p=0.68, I2=0%), BOP (χ2=0.16, p=0.68, I2=0%) or Pi (χ2=0.0007, p=0.97, I2=0%). The overall effect for PD (WMD=-0.11, 95% CI=-0.43 to 0.21, p=0.50, [Table/Fig-5a]), BOP (OR=1.03, 95% CI=0.40 to 2.62, p=0.94, [Table/Fig-5b]) and Pi (OR=0.80, 95% CI=0.29 to 2.18, p=0.66, [Table/Fig-5c]) was not statistically significant between probiotics and placebo groups at follow-up.
Forest plot presenting post-therapy clinical peri-implant parameters by comparing probiotic therapy versus placebo in patients with peri-implant mucositis.
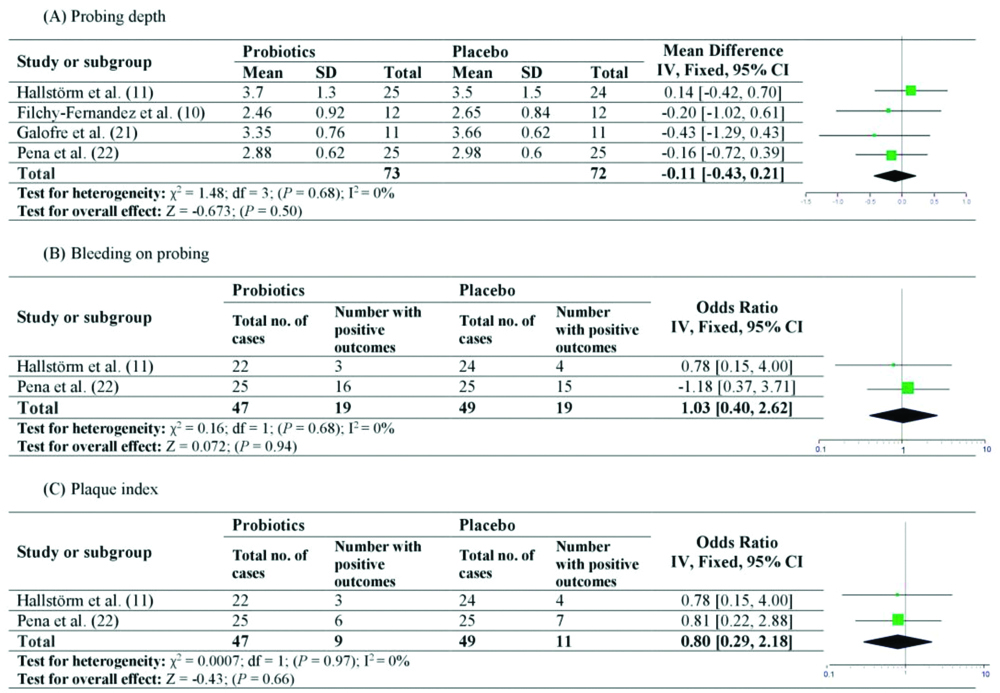
Effect of probiotics in peri-implantitis
Similarly, considering the effects of probiotics on PI, 2 studies [20,21] each for PD, BOP and Pi presented data to be included in the meta-analysis, respectively. The overall effect for PD, BOP and Pi was calculated by WMD. Fixed effect model was employed as there was no significant heterogeneity observed for PD (χ2=0.011, p=0.91, I2=0%), BOP (χ2=0.003, p=0.95, I2=0%) or Pi (χ2=0.02, p=0.87, I2=0%). The overall mean difference for PD (WMD=-0.25, 95% CI=-0.79 to 0.28, p=0.34, [Table/Fig-6a]), BOP (WMD=-0.44, 95% CI=-0.99 to 0.10, p=0.11, [Table/Fig-6b]) and Pi (WMD=-0.13, 95% CI=-0.67 to 0.40, p=0.62, [Table/Fig-6c]) was not statistically significant between probiotics and placebo groups at follow-up.
Forest plot presenting post-therapy clinical peri-implant parameters by comparing probiotic therapy versus placebo in patients with peri-implantitis.
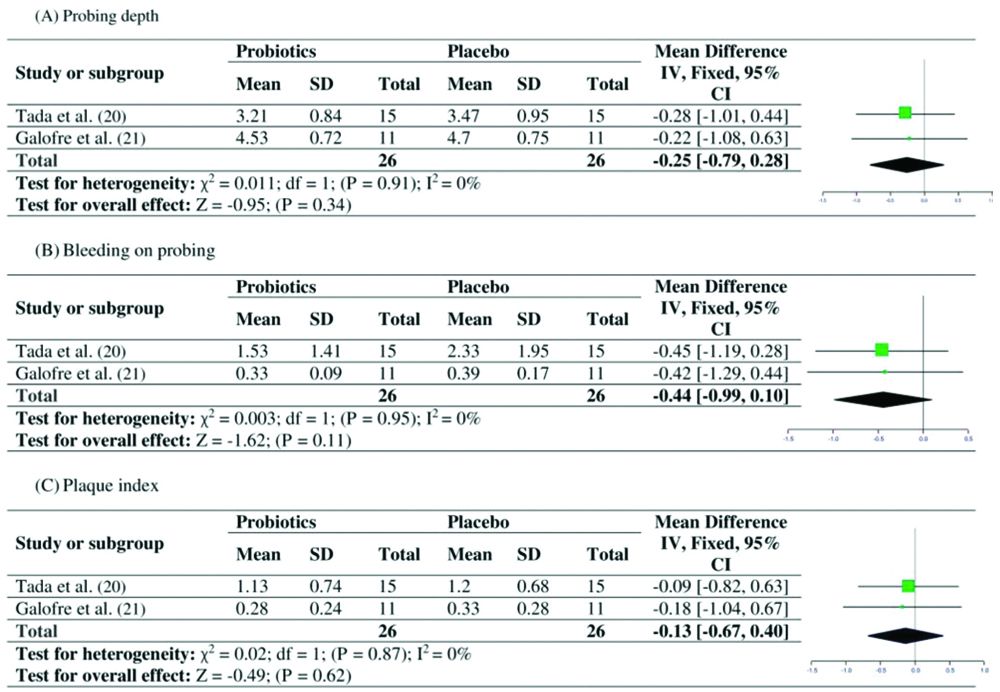
Evidence Profile
The [Table/Fig-7] showes a summary of the various factors used to rate the quality of evidence and strength of recommendations according to GRADE. Taken together, the strength of a recommendation based on the quality of the evidence emerging from this review is estimated to be moderate. Given that the effect is small, the direction of recommendation emerging from this systematic review is weak in favour of the use of probiotics in the treatment of peri-implant diseases [Table/Fig-7].
Summary of findings table on body of the estimated evidence profile and appraisal of the strength of the recommendation regarding the effectiveness of probiotics on clinical peri-implant parameters in peri-implant diseases.
| Determinants of quality | Probiotics |
|---|
| Study design | RCTs |
| Number of studies n=6 [Table/Fig-1]Comparison n=8 | 7 |
| Risk of bias | Low |
| Consistency [Table/Fig-2 and 3] | Rather not consistent |
| Directness | Generalizable |
| Precision | Rather precise |
| Publication bias (Appendices S1 and S2) | No |
| Magnitude of the effect | Small |
| Strength of the recommendation based on the body of evidence | Moderate |
| Direction of recommendation | Weak in favour of the use of probiotics |
Discussion
Probiotic administration significantly improves clinical indices, (PD, BOp and pi) compared to placebo in peri-implant diseases, this was the hypothesis of the current review. Quantitatively, no statistical significant differences in the clinical peri-implant inflammatory parameters was observed between probiotics and placebo groups at follow-up for both peri-implant mucositis and peri-implantitis, respectively.
In the studies several inconsistencies with regards to the extent of disease were noted. There was either a lack of data or dissimilar case definitions applied for the diagnosis of p-iM and/or PI which may have produced bias in the treatment outcomes. Furthermore, in both probiotic and the placebo groups a significant improvement of the gingival parameters were detected (from the observation of the included studies). Furthermore, there were significant intragroup reduction of plaque scores between both the groups. The intragroup differences between the recruitment and the baseline visits may be hypothesised by the interim positive effect of the professional instructions and cleaning at the recruitment visit and the knowledge of the patients that they are being followed-up in a study. Moreover, part of these intragroup differences may probably be also because of the Hawthorne effect [23], in which the patients may have regulated oral home care more effectively or more consistently, although the participants were asked not to change their oral hygiene habits because they knew they were being observed in the clinical studies. Although only one study evaluated the Hawthorne effect among the patients [10], future studies are warranted to assess this effect.
Most of the RCTs included in the present review used lozenges as the mode of administration of probiotic in the treatment of peri-implant diseases. These studies which used lozenges form showed comparable improvements in clinical parameters with placebo. A possible clarification regarding this may lie in the ability of systemic delivery that crosses through liver and demonstrate low availability of the drug at the target site. In contrast, the competence of subgingival drug delivery to allow high drug concentrations and have controlled long-term release of the therapeutic agents at target sites (gingival sulcus) circumventing likely systemic adverse effects [24]. Although evidence regarding patients reporting adverse effects by the use of probiotics is sparse, this could prove favourable over systemic administration due to rapid absorption and low bioavailability in the body. Future studies are warranted to compare the efficacy of systemic versus local probiotics in peri-implant inflammation.
It is well-known that certain confounding factors such as smoking or periodontal diseases affect the local inflammation around teeth and dental implants. Such modifying factors were taken into consideration in some studies. Probiotic administration showed no statistically significant improvement in the clinical inflammatory parameters in patients receiving placebo. Although the smoking dose and duration was unclear, nevertheless, no regression analysis was performed in the clinical trial to control the effect of smoking habit on peri-implant outcomes among the participants recruited which may result in significant bias in the included studies.
It is believed that true clinical benefits cannot only be gauged by clinical parameters, but it can be strengthened by incorporating surrogate measures like microbiological or immunological parameters. Although microbiological and immunological data was presented in the included studies (not reported in our systematic review); there was either a significant heterogeneity in the outcomes of these parameters, or there was a lack of quantitative data in the included studies. Such parameters could have given more evidence about the observed effect. It would be interesting to study in future trials about the colonisation and immunomodulation by probiotics in peri-implant diseases.
Less number of studies were the part of this present review which could be one of the limitations. The short follow-up duration in the included studies might not have produced in the assessed clinical indices. Furthermore, the selection criteria only considered articles in English language due to which bias may have resulted with potentially applicable studies published in other language being overlooked [25]. All these considerations, yet important factors may have caused discrepancies which make elucidations arduous and such results should be acknowledged with caution.
Conclusion
Based on the qualitative and quantitative results of this review, the efficacy of probiotics in the treatment of peri-implant diseases remains debatable. The body of evidence in the current review is limited. However, further well-designed RCTs using different strains of probiotics in broader population samples over longer periods of time are warranted to prove the effectiveness of probiotics in patients with peri-implant diseases. Furthermore, it would also be interesting to compare probiotics with different other interventions such as chlorhexidine and antibiotics.