Evaluation of Soluble Fms-like Tyrosine Kinase-1 in First Trimester of Pregnancy: A Cross-Sectional Study
Sathya Selvarajan1, Jothimalar Ramalingam2, Jaya Vijayaraghavan3, Zachariah Bobby4
1 Assistant Professor, Department of Biochemistry, Saveetha Institute of Medical and Technical Sciences, Chennai, Tamil Nadu, India.
2 Professor, Department of Biochemistry, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India.
3 Professor, Department of Obstetrics and Gynaecology, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India.
4 Professor, Department of Biochemistry, JIPMER, Puducherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Jothimalar Ramalingam, Department of Biochemistry, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai-600116, Tamil Nadu, India.
E-mail: drjothimalar@gmail.com
Introduction
Soluble fms-like tyrosine kinase-1 (sFLT-1) is the form of the Vascular Endothelial Growth Factor (VEGF) receptor-1. It is an anti-angiogenic protein tyrosine-kinase receptor that binds to the VEGF. In pregnancy, the overexpression of receptor in placenta inhibits the formation of new blood vessels in uterus, and has been implicated in abnormal embryonic and placental development, and other complications of pregnancy like preeclampsia.
Aim
To determine the levels of soluble fms-like tyrosine kinase-1 (sFLT-1) in sera of apparently healthy pregnant women in the gestational age group of 4 to 10 weeks.
Materials and Methods
A cross-sectional study conducted at Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India. Serum sFLT-1 levels of 140 pregnant women (in first trimester of their pregnancy) were measured by Enzyme-Linked Immuno Sorbent Assay (ELISA). Correlation of gestational age with sFLT-1 values was performed by using Karl Pearson’s correlation coefficient.
Results
The mean value of sFLT-1 in pregnancy was found to be 1043±435 pg/mL. A significant positive correlation was observed between gestational age and sFLT-1 in early pregnancy. Linear regression equation for predicting the sFLT-1 level based on the gestational age=282.57+94.23* (gestational age in weeks).
Conclusion
sFLT-1 levels were observed to be directly correlated to the gestational age, and increased with gestational age. Determining the level of sFLT-1 in early first trimester of pregnancy and establishing its reference interval based on gestational age can help in differentiating normal pregnancy from a non-viable pregnancy in the early stages.
Angiogenesis, Gestational age, Reference range, Vascular endothelial growth factor receptor-1
Introduction
Soluble fms-like tyrosine kinase - 1 (sFLT-1) is a protein tyrosine-kinase with anti-angiogenic characteristics. It is the soluble form of the VEGF receptor -1 which was first discovered and reported by Kendall RL and Thomas KA [1]. VEGF is a physiological regulator of angiogenesis in the skeleton, and is also known to be involved in vasculogenesis, angiogenesis, and lymphangiogenesis during embryonic and postnatal development [2]. sFLT-1 binds with both Placental Growth Factor (PlGF) and VEGF, reduces their free concentration and thus leads to inhibition of angiogenesis. Therefore, it can be postulated that sFLT-1 is a cardinal regulatory component of angiogenesis in various tissues and is implicated in numerous diseased conditions with abnormal vascular growth [3].
sFLT-1 is primarily produced in the liver, brain, kidney and placental tissues [4]. However, unlike VEGF-R1, sFLT-1 lacks the trans-membrane domain and circulates freely in the bloodstream from the site where it is secreted to the other sites where its action is exerted [3].
A crucial aspect of successful implantation of the zygote within the uterine endometrium is the establishment of angiogenesis-the physiological process of growth of new blood vessels from previously existing microvasculature involved in growth and repair. After a certain point in pregnancy, vascular transformation is necessitated rather than angiogenesis. sFLT-1 plays a major role in regulating the balance between these two processes. Although small quantities of sFLT-1 are secreted by the monocytes and endothelial cells, the placenta is the major source of protein required for foetus development [5]. sFLT-1 mRNA is strongly expressed in placenta, and levels in serum decrease significantly post-delivery [6,7]. Szalai G et al., study on mice models suggests that sFLT-1 is essential for normal embryonic development [8]. Monitoring the levels of sFLT-1 in the early first trimester especially during the first 4 to 10 weeks helps in determining the normal development and functioning of the placenta which is the prerequisite for normal embryonic development. Establishing the normal levels for this receptor can aid in early detection of complications of pregnancies. Based on these findings, the present study was conducted as an attempt to determine sFLT-1 levels in apparently normal pregnant women in their early first trimester in the gestational age group of 4 to 10 weeks. This work is part of a research on the topic to study the hormonal and other biomarkers of first trimester of pregnancy [9].
Materials and Methods
The present cross-sectional study was conducted at Sri Ramachandra Medical College Hospital & Research Institute (SRMC & RI), Chennai, Tamil Nadu from July 2017 to September 2017. The study was conducted in accordance with the recommendations of the Institutional Ethics Committee (Reference number: IEC-NI/14/OCT/43/61) and as per the guidelines of the 1964 Helsinki declaration and its later amendments. Apparently healthy 140 pregnant women (aged of 19-42 years) in 4-10 weeks of gestation visiting the OBG Department for regular check-ups were selected. The purpose of the study was explained to all the participants and written informed consent was obtained before commencement of the study. Pregnant women aged 18 years or above with serum β-hCG ≥5 IU/L with singleton normal gestation confirmed by ultrasonography and gestational age ≤10 weeks were included. Women who underwent Artificial Reproductive Therapy (ART) were excluded from the study.
Maternal age and gravidity of all subjects participating in the study were documented. Their ultrasound reports were used to identify the gestational age. Venous blood was collected by trained phlebotomist in serum separator tubes. Serum was obtained by centrifugation at 3000 rpm for 10 minutes at room temperature and stored at -20° C until further analysis. sFLT-1 was assayed using ELISA kit (R&D Systems, USA; Catalogue No. DVR100B) as per manufacturer’s instructions.
Statistical Analysis
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) software for version 16.0. The numerical data were presented as mean±standard deviation (mean±SD) and the categorical data were presented as number. Correlation of gestational age with sFLT-1 values was performed by using Karl Pearson’s correlation coefficient. and a p-value ≤0.05 was considered significant.
Results
The average maternal age, gestational age and gravidity of the pregnant women in this study are tabulated in [Table/Fig-1]. Mean sFLT-1 was found to be 1043±435 pg/mL. The minimum sFLT-1 level was 163 pg/mL and maximum was 2585 pg/mL [Table/Fig-1].
Baseline characteristics and sFLT-1 values of the study participants.
| Variable | Mean±SD | Measure range (Min-Max) |
|---|
| Maternal age (years) | 24.8±3.5 | 19-42 |
| Gestational age (weeks) | 6.3±1.6 | 4-10 |
| Gravidity | 1.4±0.5 | 1-3 |
| sFLT-1 (pg/mL) | 1043±435 | 163-2585 |
Gestational age was positively correlated with serum sFLT-1 concentration (r-value 0.549, p<0.001). A scatter-plot demonstrating the distribution of sFLT-1 values across gestational age is presented in [Table/Fig-2]. A gradational increase in sFLT-1 values along with the progression of pregnancy was observed.
Scatter plot demonstrating the distribution of sFLT-1 across gestational age.
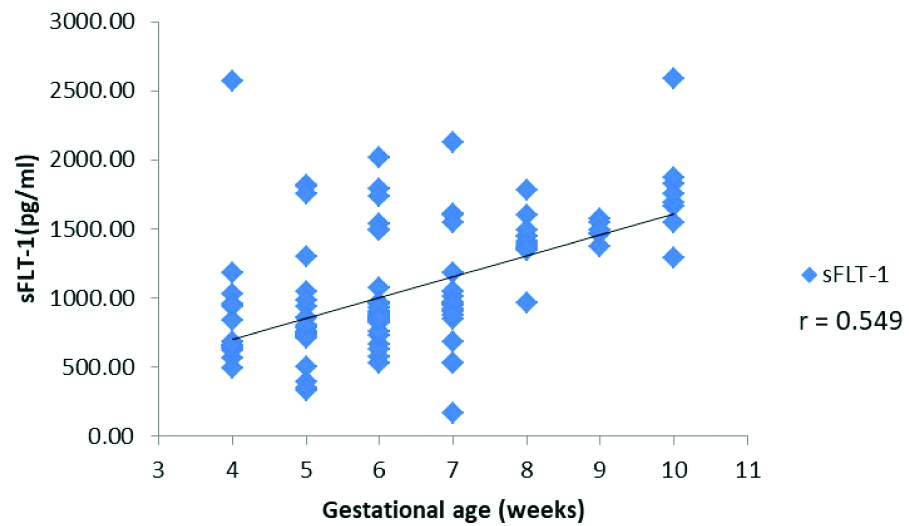
The women were divided into two groups based on their gestational age: the first group comprised of women in 4 to 6 weeks of gestation and the second group comprised of women in 7 to 10 weeks gestation. The mean, SD and range of serum FLT-1 levels are tabulated in [Table/Fig-3].
Serum sFLT-1 levels in normal pregnant women based on gestational age.
| Gestational age | sFLT-1 (pg/mL) | Normal pregnant women (n=140) |
|---|
| 4-6 weeks | N | 87 |
| Mean±SD | 893±398 |
| Range | 496-2572 |
| 7-10 weeks | N | 53 |
| Mean±SD | 1930±1593 |
| Range | 163-2585 |
Gestational week wise mean, SD and range were calculated and tabulated in [Table/Fig-4]. A multiple linear regression was calculated to predict sFLT-1 values based on gestational age. The regression equation for predicting the sFLT-1 level based on the gestational age is equal to 282.57+94.23 * (Gestational age in weeks). The calculated sFLT-1 based on the above mentioned regression formula is on the lower side of the measured mean.
Comparison of sFLT-1 across gestational age observed in the subjects.
| Gestational age (weeks) | No. of cases | Measured sFLT-1 | Calculated sFLT-1* (pg/mL) (Mean±SD) | p-value |
|---|
| Minimum | Maximum | sFLT-1 (pg/mL) (Mean±SD) |
|---|
| 4 | 15 | 496 | 2572 | 873±508 | 660 | 0.1267 |
| 5 | 34 | 332 | 1817 | 839±350 | 754 | 0.1661 |
| 6 | 38 | 526 | 2012 | 967±336 | 848 | 0.0354 |
| 7 | 25 | 163 | 2127 | 1007±390 | 942 | 0.0349# |
| 8 | 13 | 963 | 1783 | 1408±182 | 1036 | <0.0001### |
| 9 | 7 | 1372 | 1568 | 1482±70 | 1131 | <0.0001### |
| 10 | 8 | 1288 | 2585 | 1777±375 | 1225 | 0.0042## |
Measured and calculated sFLT-1 values for different gestational age (4 to 10 weeks) were compared and p-values calculated using student t-test; *Regression equation for sFLT-1= 282.57+94.226 * (gestational age in weeks); # Statistically significant; ## Very statistically significant; ###Extremely statistically significant
Discussion
In normal pregnancy, enormous quantities of VEGF are secreted from macrophages at the Nitabuch’s stria of decidua during the first trimester of pregnancy [10]. Here vascular transformation is necessitated instead of angiogenesis, and a balance between angiogenesis and vascular transformation is essential. sFLT-1 is produced by the trophoblastic cells of the placenta which are positioned between the mother’s blood vessels on one side and the umbilical vessels on the side of the foetus [11]. sFLT-1 binds with both VEGF and PlGF, forming a barricade in opposition to atypical vascular penetrability and aberrant angiogenesis, and prevents merging of foetal blood vessels to maternal capillaries. The trophoblastic villi have an uninterrupted communication with maternal circulation within the placenta and therefore, the proteins produced there can be identified in maternal blood. These sFLT-1 levels are insignificantly low in men and non-pregnant women where sFLT-1 are expressed by tissues other than placenta [12]. Since there is a significant increase in placental size and villous trophoblast in the course of pregnancy, it is to be expected that the overall sFLT-1 production will increase [13].
Futhermore sFLT-1 is a marker of angiogenesis, it is expected to be altered in non-viable Intrauterine Pregnancy (IUP) [10]. Variation in blood levels of this receptor has been implicated in failing and abnormal pregnancies like ectopic pregnancy and preeclampsia. Muttukrishna S et al., reported similar findings presenting that sFLT-1 levels in early pregnancy are inversely correlated to the level of oxygen in the placental blood [14].
Various studies suggest that an uncharacteristic raise in the level of serum sFLT-1 in expectant mothers is associated with the degree of preeclampsia [5,8,1516]. sFLT-1 released from the placenta travels in the mother’s circulation to distant target organs and are responsible for the multisystem endothelial dysfunction in preeclampsia [15]. Mice models also reports overexpression of sFLT-1 levels in implication of preeclampsia [8]. While another study reported that reduction in sFLT-1 level was found to alleviate preeclampsia-like symptoms in mouse model [16].
Since the implantation of an Ectopic Pregnancy (EP) at the tubal site is unfavourable, it provides an abnormal environment with insufficient nutrition and oxygen to the developing embryo. This hypoxic environment increases the expression of VEGF in the ectopic site. It has been reported that VEGF in serum of women with EP is elevated in comparison with Intra-Uterine Pregnancy IUP [17]. There is also a reduction in the levels of sFLT-1 in EP women however; the exact mechanism of reduction of sFLT-1 levels remains unclear. Measurement of sFLT-1 therefore aids in identifying implantation of the embryo at an ectopic site and can be used as an early biomarker for EP [18,19].
In the present study, the mean value of sFLT-1 in pregnant women in the gestational age of 4-10 weeks was found to be 1043 pg/mL. These findings were in accordance with Daponte A et al., results, who reported the median sFLT-1 in normal pregnant women as 1390.32 pg/mL [18]. In contrast, the sFLT-1 values reported by Martínez-Ruiz A et al., were lower at 505 (121-945) pg/mL [19]. The difference in the reported value can be accounted to different population (s) studied. Statistically significant positive correlation was observed between gestational age and sFLT-1 levels (r-value 0.549). Similar positive correlation was reported by Kim SY et al., and Wataganara T et al., [20,21].
Although a transvaginal ultrasound can detect a gestational sac as early as 5 weeks; the foetal pole visualised at 6 weeks and the foetal heart beat is picked up by around 7 weeks of gestation, 5-42% of the ultrasound are inconclusive at first presentation [22]. The initial 4-6 weeks of conception are therefore critically important from a diagnostic viewpoint, to determine the presence of an extra-uterine implantation of blastocyst or a non-viable pregnancy. In the present study, pregnant women were divided into two groups based on gestational age: 4-6 weeks and 7-10 weeks. The measured sFLT levels in the former group was 893 pg/mL and the latter was 1930 pg/mL. In the present study, Maternal age (years), gestational age (weeks) and gravidity were not found to influence sFLT-1 concentration, as also evidenced by Tsiakkas A et al., [23].
A multiple linear regression was performed to predict sFLT-1 values based on gestational age. The regression equation for predicting the sFLT-1 level based on the gestational age was derived to be 282.57+94.226 * (gestational age in weeks). sFLT-1 increased by 94.226 pg/mL in addition to the constant factor of 282.57 for each gestational week from 4 to 10 weeks in normal pregnancy. So the calculated expected values for a particular gestational age can be compared with the measured values in pregnant women with suspicion of a non-viable or failing pregnancy to rule out the same. The study by Richardson A et al., evaluated the association of sFLT-1 with viability of pregnancy [24]. A recent study by Cerdeira AS et al., confirmed the main source of sFLT-1 in pregnancy to be the placenta, furthering evidence on our hypothesis [25].
Limitation
Relatively small sample size and uneven number of cases in different gestational age groups are few limitations of the present study. Further cohort studies are required to establish gestational age-specific reference range for early diagnosis of abnormal/ failing pregnancies.
Conclusion
sFLT-1 levels in pregnancy helps in establishment of a balance between angiogenesis and vascular transformation, and concentration of sFLT-1 gives an overview of location of placentation in pregnancy. Therefore, establishing the normal reference range can help in early diagnosis of complication of pregnancies like ectopic pregnancy, preeclampsia and other failing pregnancies.
Measured and calculated sFLT-1 values for different gestational age (4 to 10 weeks) were compared and p-values calculated using student t-test; *Regression equation for sFLT-1= 282.57+94.226 * (gestational age in weeks); # Statistically significant; ## Very statistically significant; ###Extremely statistically significant
Author Declaration:
Financial or Other Competing Interests: Yes (as declared above)
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jul 27, 2019
Manual Googling: Oct 10, 2019
iThenticate Software: Nov 19, 2019 (13%)
[1]. Kendall RL, Thomas KA, Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptorProceedings of the National Academy of Sciences of the United States of America 1993 90(22):10705-09.10.1073/pnas.90.22.107058248162 [Google Scholar] [CrossRef] [PubMed]
[2]. Ho QT, Kuo CJ, Vascular endothelial growth factor: biology and therapeutic applicationsInt J Biochem Cell Biol 2007 39(7-8):1349-57.10.1016/j.biocel.2007.04.01017537667 [Google Scholar] [CrossRef] [PubMed]
[3]. Lecarpentier E, Tsatsaris V, Angiogenic balance (sFlt-1/PlGF) and preeclampsiaAnnales d’endocrinologie 2016 77(2)Elsevier Masson:97-100.10.1016/j.ando.2016.04.00727130072 [Google Scholar] [CrossRef] [PubMed]
[4]. Fan X, Rai A, Kambham N, Sung JF, Singh N, Petitt M, Endometrial VEGF induces placental sFLT1 and leads to pregnancy complicationsJ Clin Invest 2014 124:4941-52.10.1172/JCI7686425329693 [Google Scholar] [CrossRef] [PubMed]
[5]. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsiaThe Journal of Clinical Investigation 2003 111(5):649-58.10.1172/JCI1718912618519 [Google Scholar] [CrossRef] [PubMed]
[6]. Andraweera PH, Dekker GA, Laurence JA, Roberts CT, Placental expression of VEGF family mRNA in adverse pregnancy outcomesPlacenta 2012 33:467-72.10.1016/j.placenta.2012.02.01322386962 [Google Scholar] [CrossRef] [PubMed]
[7]. Tang Y, Ye W, Liu X, Lv Y, Yao C, Wei J, VEGF and sFLT-1 in serum of PIH patients and effects on the foetusExperimental and Therapeutic Medicine 2019 17(3):2123-28.10.3892/etm.2019.718430867699 [Google Scholar] [CrossRef] [PubMed]
[8]. Szalai G, Xu Y, Romero R, Chaiworapongsa T, Xu Z, Chiang PJ, In Vivo experiments reveal the good, the bad and the ugly faces of sFlt-1 in pregnancyPLoS ONE 2014 9(11):e11086710.1371/journal.pone.011086725393290 [Google Scholar] [CrossRef] [PubMed]
[9]. Selvarajan S, Jothimalar R, Serum progesterone in first trimester of normal pregnancyInt J Clin Biochem Res 2018 5(4):651-53.10.18231/2394-6377.2018.0138 [Google Scholar] [CrossRef]
[10]. Zarezade N, Saboori Darabi S, Ramezanali F, Amirchaghmaghi E, Khalili G, Moini A, mRNA expression of VEGF and its receptors in fallopian tubes of women with ectopic pregnanciesInternational Journal of Fertility & Sterility 2015 9(1):55-64. [Google Scholar]
[11]. Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion?The Journal of Clinical Investigation 2007 99(9):2139-51.10.1172/JCI1193879151786 [Google Scholar] [CrossRef] [PubMed]
[12]. Weissgerber TL, Rajakumar A, Myerski AC, Edmunds LR, Powers RW, Roberts JM, Vascular pool of releasable soluble VEGF receptor-1 (sFLT1) in women with previous preeclampsia and uncomplicated pregnancyJ Clin Endocrinol Metab 2014 99(3):978-87.10.1210/jc.2013-327724423299 [Google Scholar] [CrossRef] [PubMed]
[13]. Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, A Vascular Endothelial Growth Factor Antagonist Is Produced by the Human Placenta and Released into the Maternal CirculationBiology of Reproduction 1998 59(6):1540-48.10.1095/biolreprod59.6.15409828203 [Google Scholar] [CrossRef] [PubMed]
[14]. Muttukrishna S, Swer M, Suri S, Jamil A, Calleja-Agius J, Gangooly S, Soluble Flt-1 and PlGF: New Markers of Early Pregnancy Loss?PLoS ONE 2011 6(3):e1804110.1371/journal.pone.001804121448460 [Google Scholar] [CrossRef] [PubMed]
[15]. Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA, Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsiaPediatric Research 2005 57(5 Pt 2):1R-7R.10.1203/01.PDR.0000159567.85157.B715817508 [Google Scholar] [CrossRef] [PubMed]
[16]. Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse modelJ Cell Mol Med 2010 14(6B):1857-67.10.1111/j.1582-4934.2009.00820.x19538465 [Google Scholar] [CrossRef] [PubMed]
[17]. Felemban A, Sammour A, Tulandi T, Serum vascular endothelial growth factor as a possible marker for early ectopic pregnancyHum Reprod 2002 17(2):490-92.10.1093/humrep/17.2.49011821301 [Google Scholar] [CrossRef] [PubMed]
[18]. Daponte A, Pournaras S, Polyzos NP, Tsezou A, Skentou H, Anastasiadou F, Soluble fms-like tyrosine kinase-1 (sFlt1) and serum placental growth factor (PlGF) as biomarkers for ectopic pregnancy and missed abortionJ Clin Endocrinol Metab 2011 96(9):1444-51.10.1210/jc.2011-003721715541 [Google Scholar] [CrossRef] [PubMed]
[19]. Martínez-Ruiz A, Sarabia-Meseguer MD, Pérez-Fornieles J, Vílchez JA, Tovar-Zapata I, Noguera-Velasco JA, Placental growth factor, soluble fms-like tyrosine kinase 1 and progesterone as diagnostic biomarkers for ectopic pregnancy and missed abortionClin Biochem. The Canadian Society of Clinical Chemists 2014 47(9):844-47.10.1016/j.clinbiochem.2014.03.01324699433 [Google Scholar] [CrossRef] [PubMed]
[20]. Kim SY, Ryu HM, Yang JH, Kim MY, Han JY, Kim JO, Increased sFlt-1 to PlGF ratio in women who subsequently develop preeclampsiaJournal of Korean Medical Science 2007 22(5):873-77.10.3346/jkms.2007.22.5.87317982238 [Google Scholar] [CrossRef] [PubMed]
[21]. Wataganara T, Pratumvinit B, Lahfahroengron P, Pooliam J, Talungchit P, Leetheeragul J, Circulating soluble fms-like tyrosine kinase-1 and placental growth factor from 10 to 40 weeks’ pregnancy in normotensive womenJ Perinat Med 2017 45(7):895-901.10.1515/jpm-2017-009328665791 [Google Scholar] [CrossRef] [PubMed]
[22]. Kirk E, Bottomley C, Bourne T, Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown locationHum Reprod Update 2014 20:250-61.10.1093/humupd/dmt04724101604 [Google Scholar] [CrossRef] [PubMed]
[23]. Tsiakkas A, Duvdevani N, Wright A, Wright D, Nicolaides KH, Serum soluble fms-like tyrosine kinase-1 in the three trimesters of pregnancy: effects of maternal characteristics and medical historyUltrasound Obstet Gynecol 2015 45(5):584-90.10.1002/uog.1481725678265 [Google Scholar] [CrossRef] [PubMed]
[24]. Richardson A, Deb S, Campbell B, Raine-Fenning N, Serum concentrations of Ang-2 and Flt-1 may be predictive of pregnancy outcome in women with pregnancies of uncertain viability: a phase I exploratory prognostic factor studyJournal of Obstetrics and Gynaecology 2018 38(3):321-26.10.1080/01443615.2017.135359629072547 [Google Scholar] [CrossRef] [PubMed]
[25]. Cerdeira AS, Kandzija N, Pargmae P, Cooke W, James T, Redman C, Circulating soluble fms-like tyrosine kinase-1 is placentally derived in normal pregnancy: First in vivo evidencePregnancy hypertension 2019 16:145-47.10.1016/j.preghy.2019.03.01331056150 [Google Scholar] [CrossRef] [PubMed]