Comparative Study of Two Different Intravenous Doses of Tranexamic Acid with Placebo on Surgical Field Quality in Functional Endoscopic Sinus Surgery- A Randomised Clinical Trial
Tamil Anbu Pannerselvam1, Kodali V Rajesh Kumar2, Ranjith B Karthekeyan3
1 Assistant Professor, Department of Emergency Medicine, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India.
2 Associate Professor, Department of Anaesthesiology, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India.
3 Professor, Department of Cardiac Anaesthesiology, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Kodali V Rajesh Kumar, Flat No. F20, SRMC Staff Quarters, F Block, Porur, Chennai, Tamil Nadu, India.
E-mail: vrajesh.kodali@gmail.com
Introduction
Intraoperative haemorrhage, which disrupts surgical patency, is a major obstacle for surgeons, especially in endoscopic sinus surgeries. Tranexamic acid is synthetic antifibrinolytic agent that binds competitively at lysine binding site on plasminogen prevents fibrinolysis, improves blood clot formation and reduces bleeding. The role of tranexamic acid by topical adminstration during Functional Endoscopic Sinus Surgeries (FESS) had contrasting reports and limited number of studies are available with systemic adminstration of tranexamic acid in FESS surgeries.
Aim
To compare two different intravenous doses of tranexamic acid with placebo on surgical field quality, surgical time and blood loss in FESS surgeries.
Materials and Methods
Eighty four ASA physical status I and II patients from January 2013 to December 2013 aged 18-60 years, who underwent FESS surgery, were included in the study. Patients were randomised to Group A receiving 15 mg.kg-1 tranexamic acid Group B receiving 5 mg.kg-1 tranexamic acid and Group C as placebo receiving normal saline. Heart rate, systolic, diastolic blood pressure, oxygen saturation, blood loss and surgical site quality were recorded. Statistical analysis between treatment groups was performed using repeated measures of ANOVA and intergroup analysis was performed where appropriate. The primary outcome was surgical field quality; secondary outcome was blood loss and surgical time.
Results
Intravenous administration of tranexamic acid was found to reduce the total blood loss in FESS surgery by 64% in group A (15 mg.kg-1) and 31% in group B (5 mg.kg-1) compared to placebo (p=0.0001). Reduction in bleeding by administration of tranexamic acid also led to an improved surgical field quality. There was partial surgical field clearance at a low dose of tranexamic acid while 15 mg.kg-1 of the drug achieved about 100% surgical site clearance as measured with Wormald Grading Scale of surgical field quality (p=0.0001). This was also validated by a surgeon satisfaction score after FESS procedure using Likert scale and the fraction of patients for whom surgery was affected by bleeding. The surgical field improvement by use of tranexamic acid has also been found to have a prominent reduction in the total time taken to complete the surgery. Administration of 5 mg.kg-1 drug led to a 7% reduction and 15 mg.kg-1 of tranexamic acid led to about 15% reduction in time taken for FESS surgery (p=0.0001).
Conclusion
Tranexamic acid 15 mg/kg dose effectively reduces blood loss, improves surgical field quality and reduces surgical time.
Antifibrinolytic, Intraoperative bleeding, Surgical field quality
Introduction
Patients reporting with recurrent acute or chronic sinusitis and nasal polyps are considered for FESS with above 90% recovery rate [1-4]. Intraoperative bleeding obstructing surgical field visibility is a common challenge faced by surgeons performing FESS. Many anaesthetics, vasodilators (sodium nitroprusside, nitroglycerine), alpha and beta-adrenergic receptor blockers, calcium channel blockers, prostaglandin E1, etc., are used to facilitate controlled bleeding during surgery. These drugs act on peripheral vessels and are known to cause hypotension, reduced tissue perfusion and acidosis [4-8].
Antifibrinolytics are a class of drugs that inhibit the fibrinolytic pathway in tissue injury and have been used effectively to reduce post-operative bleeding and traumatic bleeding [9-14]. Tranexamic acid administration at the end of procedures had not shown to reduce bleeding [15] Topical administration of tranexamic acid had contrasting reports [16-18]. Only few studies compared intra venous infusion of tranexamic acid 10 mg/kg with ethamsylate or placebo [19-21]. Dongare VR et al., compared 15 mg/kg tranexamic acid infusion with placebo while Langille MA et al., compared tranexamic acid bolus dose of 15 mg/kg followed by infusion dose of 1 mg/kg/hr with saline [22,23]. Abbasi HA et al., compared 2 doses of tranexamic acid with Boezart scale [24]. Most of the studies were conducted with single dose infusion compared with placebo.
Hence the current study aimed to compare two different intravenous doses of tranexamic acid with placebo on the surgical field quality, surgical time and blood loss with in FESS surgeries.
Materials and Methods
The study was designed as a randomised, double-blinded clinical trial after approval (SRIHER/FEB/2019/PP012) from the Institutional Ethical Committee. Patients who came from January 2013 to December 2013 under class I and II of American Society of Anaesthesiologists’ (ASA) physical status, undergoing functional endoscopic sinus surgery and of age group 18-60 years from both sex, were included in the study. Patients having features such as ASA class III and IV, contraindication to the study drug (Tranexamic acid), thrombotic diathesis, vascular disease and renal failure were excluded from the study.
Sample size was calculated based on power analysis (Power of 80%, α error 5%, mean difference: 0.63; effect size: 0.763; two-sided test) done on the surgical field quality assessed by Boezart scale of previous study at 120 min in patients undergoing FESS and receiving Tranexamic acid [20]. nMaster 2.0 software was used for the analysis.
Procedure
The patients were screened; the pre-operative anaesthetic examination was performed and enrolled in the study after acquiring informed consent [Table/Fig-1]. The patients were randomised by computer generated block randomization into Group A (n=28, tranexamic acid dose-15 mg Kg-1), Group B (n=28, tranexamic acid dose-5 mg Kg-1) and Group C (control group, normal saline n=28). Patients who participated in the study were not prescribed with pre-anaesthetic medications and were fasted from midnight prior to the surgery. Study drug prepared in 100 mL Normal Saline (NS) according to group assigned by independent anesthesiologist not participating in the study and was administered to the patients 20 minutes before surgery in the pre-operative holding area. The personnel involved in surgery including anaesthesiologists, surgeons, nurses and other staff blinded to study protocol.
Consort flow chart of the study.
Patient’s baseline Electrocardiogram (ECG), Heart Rate (HR), Systolic (SBP), Diastolic (DBP) blood pressures, oxygen saturation (SPO2) monitors were connected once the patient enter into the operating room. IV access was secured, and ringer lactate solution was administered according to fluid administration guidelines [25]. Patients undergoing the procedure were preoxygenated at 6 Lmin-1 for 3 mins (100% oxygen). Anaesthesia was induced by administering Midazolam (1 mg Kg-1), Fentanyl (2 μg kg-1), Propofol (2 mg Kg-1) and Vecuronium (0.1 mg Kg-1). Patients were intubated after 3 minutes of induction and positioned 15° head-up before the start of surgery. Anaesthesia was maintained using Nitrous Oxide- 2 Lmin-1, Oxygen- 1 L min-1 and 1 MAC of sevoflurane and ETCO2 level maintained at 30 to 35 mmHg. Intraoperatively HR, SBP, DBP, SPO2 were monitored every 3 minutes till the end of procedure. All the patients were given neostigmine 0.05 mg Kg-1 and glycopyrrolate 0.01 mg Kg-1 to reverse neuromuscular blockade and were extubated at the end of procedure. Postoperatively heart rate, systolic and diastolic blood pressures, O2 saturation were monitored every 15 minutes for 60 minutes in the PACU. Primary outcome assessed was surgical field quality assessed by Wormald grading scale. Secondary outcomes assessed were total blood loss, surgical time and surgeons satisfaction score which was assessed by likerts scale.
Investigations
Intraoperative HR, SBP, DBP, SPO2 were noted at 30, 60, 90 minutes. Intraoperative blood loss was measured by estimating total blood from the suction bottles, tubings and the weight difference between soaked gauze, cotton ball, patty, throat pack and dry gauze, at the end of procedure. Surgical field quality was assessed by Wormald Grading Scale as it was found superior to Boezart Scale [26] [Table/Fig-2]. Surgeon satisfaction was assessed using Likert Scale 1-5 (very bad, bad, average, good, and excellent). Total blood loss and surgical field clarity were compared among the three groups.
Wormald grading scale of surgical field quality [26].
| Grade | Assessment |
|---|
| 0 | No bleeding |
| 1 | 1-2 points of ooze |
| 2 | 3-4 points of ooze |
| 3 | 5-6 points of ooze |
| 4 | 7-8 points of ooze |
| 5 | 9-10 points of ooze |
| 6 | 10 points of ooze obscuring surface |
| 7 | Mild bleeding or oozing from entire surgical surface with slow accumulation of blood in post nasal space |
| 8 | Moderate bleeding from entire surgical surface with moderate accumulation of blood in post-nasal space |
| 9 | Moderately severe bleeding with rapid accumulation of blood in the post nasal space |
| 10 | Severe bleeding with nasal cavity filling rapidly |
Statistical Analysis
All statistical analysis were performed using Statistical Package for Social Science (SPSS, version 17) for Microsoft windows. The data were normally distributed and therefore parametric tests were performed. Descriptive statistics were presented as numbers and percentages. The data were expressed as Mean and SD. A repeated measures analysis of variance (ANOVA) with a post-hoc Tukey HSD was used. Pearson’s Chi-square test was used to evaluate whether distributions of categorical variables differed from one another. A two sided p-value <0.05 was considered statistically significant.
Results
The demographic data of study population are comparable between the study groups and placebo with p-value more than 0.05 [Table/Fig-3]. During the FESS procedure, tranexamic acid did not cause statistically significant change in heart rate systolic and diastolic blood pressures within groups at 30, 60 and 90 minutes (p-value more than 0.05. Heart rate change was not statistically significant between groups and with in groups. Systolic blood pressure change was not statistically significant between the groups. Systolic blood pressure within groups was statistically significant (p-value 0.005). Diastolic blood pressure change was statistically significant between groups (p-value 0.015) and with in groups (p-value 0.001) [Table/Fig-4a,b]. Post-hoc analysis with Tukey HSD shows no statistical significant difference between heart rate and systolic blood pressures. Diastolic blood pressure in group B was less than group C which was statistically significant in post-hoc analysis.
Patient demographic data showing American Society of Anesthesiologists (ASA) classification of patients involved in the study (n=28 in each group); Age group of patients enrolled in the study and Gender.
| Group A | Group B | Group C | p-value |
|---|
| Age (years) Mean (SD) | 32.79 (10.48) | 31.68 (9.59) | 33.00 (11.25) | 0.880 |
| Weight (kgs) Mean (SD) | 61.36 (6.98) | 61.11 (8.39) | 57.57 (10.29) | 0.194 |
| Gender |
| Males | 22 | 18 | 15 | 0.142 |
| Females | 6 | 10 | 13 |
| ASA category |
| ASA 1 | 23 | 22 | 24 | 0.784 |
| ASA 2 | 5 | 6 | 4 |
Comparison of haemodynamics with administration of Tranexamic acid (Heart Rate, Systolic and Diastolic Blood Pressure) during FESS surgery (Graphical Representation).
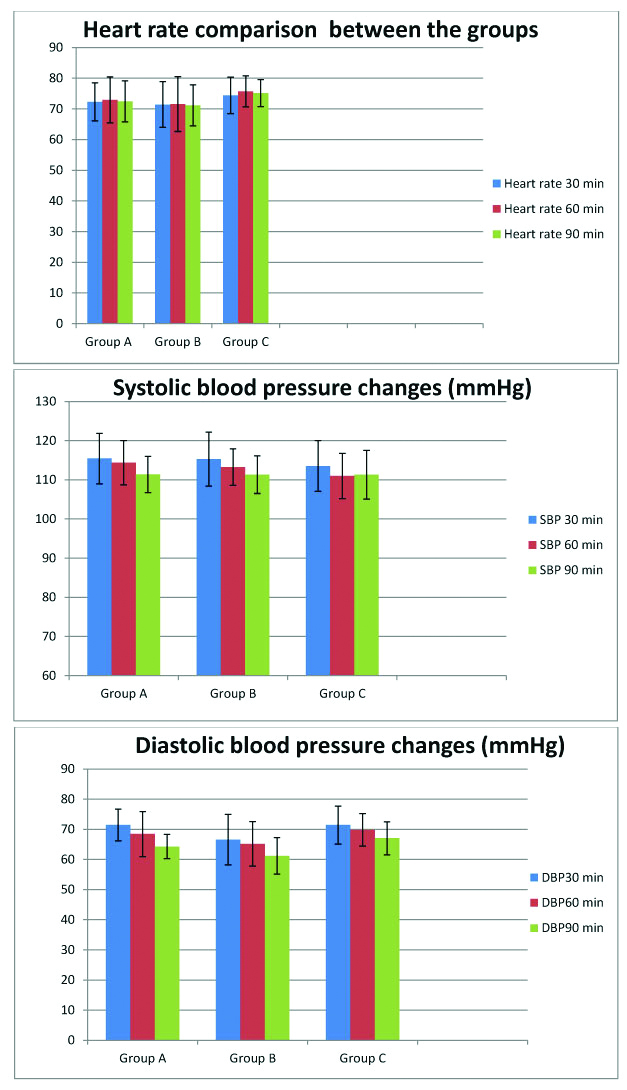
Comparison of haemodynamics with administration of Tranexamic acid (Heart Rate, Systolic and Diastolic Blood Pressure) during FESS surgery (Represented as Mean (SD) and p-value).
| Groups | 30 min | 60 min | 90 min | p-value between the groups | p-value with in the groups | p-value interaction (effect due to tranexemic acid) |
|---|
| Heart rate mean (sd) | Group A | 72.29 (6.219) | 72.93 (7.47) | 72.43 (6.64) | 0.058 | 0.551 | 0.974 |
| Group B | 71.40 (7.45) | 71.56 (8.95) | 71.16 (6.67) |
| Group C | 74.36 (5.96) | 75.71 (5.08) | 75.11 (4.40) |
| Systolic blood pressure mean (sd) | Group A | 115.43 (6.44) | 14.36 (5.66) | 111.36 (4.63) | 0.40 | 0.005 | 0.377 |
| Group B | 115.26 (6.89) | 113.26 (4.65) | 111.30 (4.79 |
| Group C | 113.50 (6.50) | 110.97 (5.76) | 111.32 (6.23) |
| Diastolic blood pressure mean (sd) | Group A | 71.43 (5.25) | 68.43 (7.47) | 64.29 (4.00) | 0.015 | 0.001 | 0.29 |
| Group B | 66.57 (8.37) | 65.17 (7.37) | 61.19 (6.06) |
| Group C | 71.43 (6.31) | 69.79 (5.71) | 67.04 (5.47) |
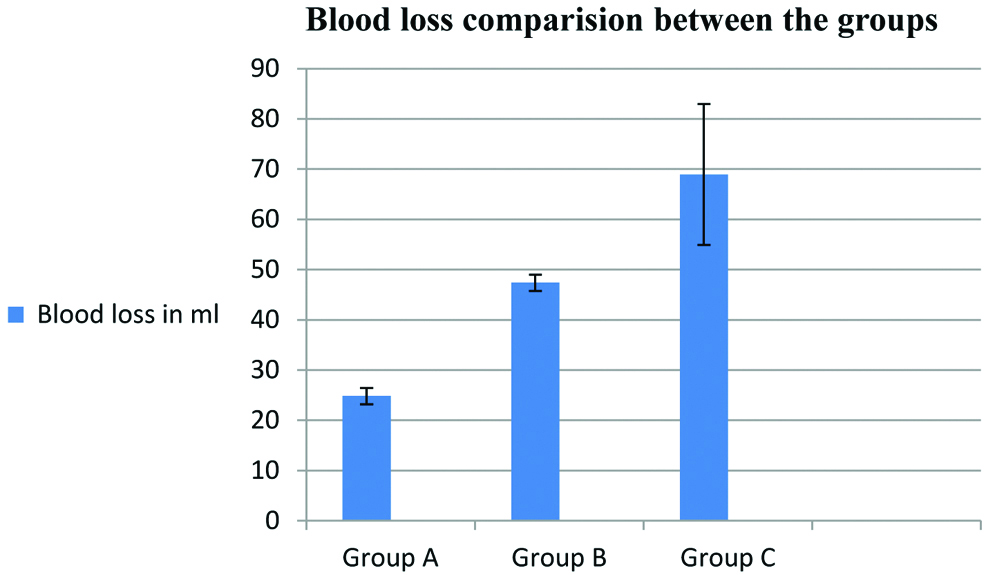
Intravenous administration of tranexamic acid was found to reduce the total blood loss in FESS surgery by 64% in group A (15 mg Kg-1) and 31% in group B (5 mg Kg-1) compared to placebo (p=0.0001) [Table/Fig-5a,b).
Blood loss comparison between the groups represented as Mean (SD).
| | Mean | SD | df | F | p-value |
|---|
| Total blood loss (mL) | Group A | 24.82 | 1.61 | Between groups 2 | 202.11 | 0.001 |
| Group B | 47.39 | 1.61 |
| Group C | 68.93 | 14.03 | With in groups 81 |
Blood loss comparison between the groups.
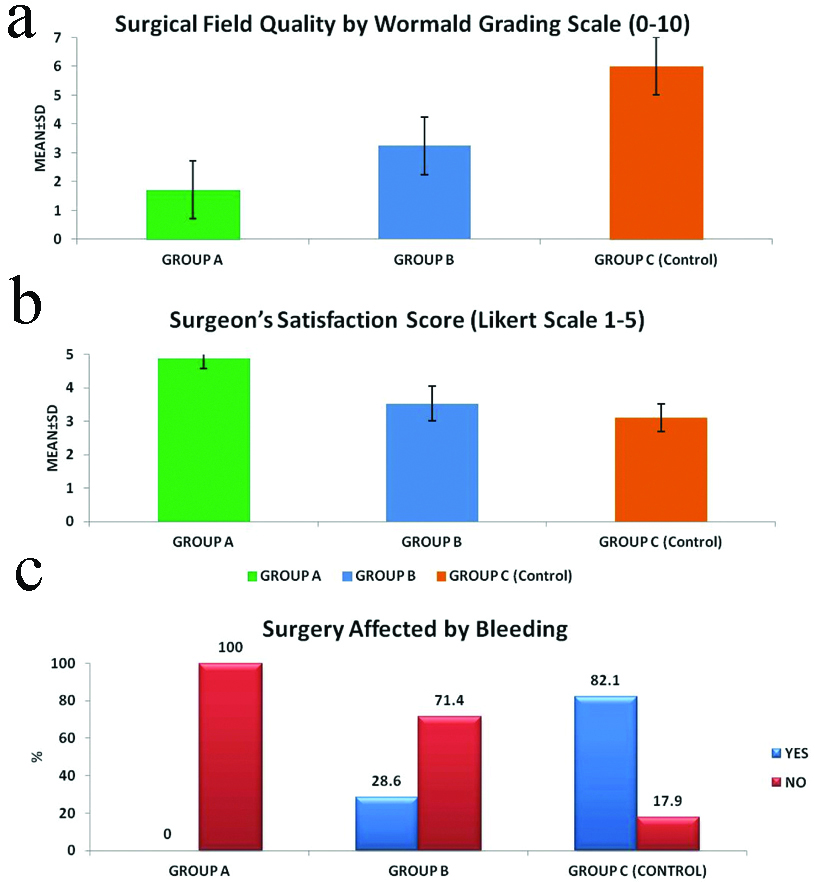
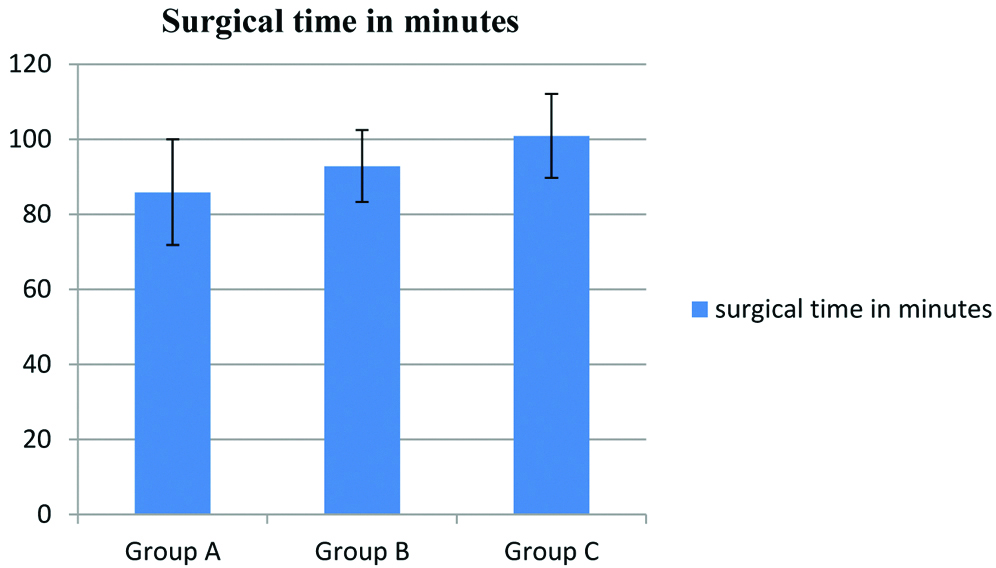
Reduction in bleeding by administration of tranexamic acid also led to an improved surgical field quality. There was partial surgical field clearance at a low dose of tranexamic acid while 15 mg Kg-1 of the drug achieved about 100% surgical site clarity as measured with Wormald Grading Scale of surgical field quality (p=0.0001) [Table/Fig-6a]. This was also validated by a surgeon satisfaction score after FESS procedure using Likert scale and the fraction of patients for whom surgery was affected by bleeding [Table/Fig-6b,c]. The surgical field improvement by use of tranexamic acid has also been found to have a prominent reduction in the total time taken to complete the surgery. Administration of 5 mg Kg-1 drug led to a 7% reduction and 15 mg Kg-1 of tranexamic acid led to about 15% reduction in time taken for FESS surgery (p=0.0001) [Table/Fig-7].
Surgical field quality obtained by administration of tranexamic acid (n=28 in each group): a) Surgical Field Quality by Wormald Grading Scale: b) Surgeon satisfaction score by Likert Scale: c) Fraction of patients for whom surgery was affected by bleeding.
Reduction in blood loss by tranexamic acid administration significantly reduces total time taken for FESS Surgery (n=28, * p<0.05).
| | Mean | Standard deviation | Df | F | p-value |
|---|
| Surgical time in minutes | Group A | 85.89 | 14.08 | Between groups- 2 | 11.38 | 0.01 |
| Group B | 92.86 | 9.56 |
| Group C | 100.89 | 11.22 | With in groups- 81 |
 |
Discussion
The current study has shown that tranexamic acid (at 15 mg Kg-1) significantly reduced total blood loss during functional endoscopic sinus surgery up to 64%. A previous study has also shown reduction in blood loss up to about 50% with tranexamic acid at 10 mg Kg-1 [27] and this index study has shown that an increased dose of 15 mg Kg-1 could help in further reduction of blood loss and hence could be effectively used. The reduced blood loss also helped to achieve improved surgical field quality as shown with the WORMALD scale of surgical field clearance. The improved surgical field further helped in reducing the total duration of the FESS procedure by 14%.
A comparative study showed that both 100 mg Kg-1 Epsilon Aminocaproic Acid and 10 mg Kg-1 tranexamic acid reduced blood loss and improved surgical field in FESS surgery in a similar manner [19]. Alimian M and Mohseni M, showed that 10 mg Kg-1 tranexamic acid reduced bleeding during FESS surgery [21] and Dongare VR and Saundattikar GY, Langille MA et al., Nuhi S et al., showed that a single bolus dose of 15 mg/kg of tranexamic acid reduces bleeding, improves surgical field quality and reduction in operating time in FESS procedure [22,23,28]. Study done by Dunn CJ and Goa KL, showed that rapid administration of tranexamic acid leads to hypotension and other complications like nausea and vomiting [29]. In this study, we did not had much hypotension due to tranexamic acid alone. Significant difference of diastolic blood pressure in group B is due to few patients had baseline low diastolic blood pressure compared to other groups.
Tranexamic acid has higher antifibrinolytic activity than its analogues and also has been listed as one of the essential medicines by the World Health Organisation (WHO) and is also available at low cost. Hence use of tranexamic acid (15 mg Kg-1) through intravenous route prior to FESS surgery is highly favourable.
Limitation
We did not document baseline haemodynamics data before adminstration of tranexamic acid. We also did not compare baseline haemoglobin and haemoglobin level at the end of surgery between three groups which also had impact on diastolic blood pressure.
Conclusion
It can be concluded that intravenous Tranexamic acid at a dose of 15 mg Kg-1 offers better surgical field quality and reduced blood loss in FESS than at a dose of 5 mg Kg-1 and placebo.
Author Declaration:
Financial or Other Competing Interests: No
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Oct 09, 2019
Manual Googling: Nov 05, 2019
iThenticate Software: Nov 19, 2019 (11%)
[1]. Gohar MS, Niazi SA, Niazi SB, Functional Endoscopic Sinus Surgery as a primary modality of treatment for primary and recurrent nasal polyposisPak J Med Sci 2017 33(2):380-82.10.12669/pjms.332.1180028523041 [Google Scholar] [CrossRef] [PubMed]
[2]. Venkatachalam VP, Bhat A, Functional endoscopic sinus surgery- a newer surgical concept in the management of chronic sinusitisIndian J Otolaryngol Head Neck Surg 1999 52(1):13-16. [Google Scholar]
[3]. Mafee MF, Chow JM, Meyers R, Functional endoscopic sinus surgery: anatomy, CT screening, indications, and complicationsAm J Roentgenol 1993 160:735-44.10.2214/ajr.160.4.84566548456654 [Google Scholar] [CrossRef] [PubMed]
[4]. Al-Mujaini A, Wali U, Alkhabori M, Functional endoscopic sinus surgery: Indications and complications in the ophthalmic fieldOman Med J 2009 24(2):70-80. [Google Scholar]
[5]. Cinčkas D, Ivaškevičius J, Martinkėnas JL, Balseris S, A role of anesthesiologist in reducing surgical bleeding in endoscopic sinus surgeryMedicina (Kaunas) 2010 46(11):730-34.10.3390/medicina46110103 [Google Scholar] [CrossRef]
[6]. Prasant MC, Kar S, Rastogi S, Hada P, Ali FM, Mudho A, Comparative study of blood loss, quality of surgical field and duration of surgery in maxillofacial cases with and without hypotensive anesthesiaJ Int Oral Health 2014 6(6):18-21. [Google Scholar]
[7]. Manjuladevi M, Vasudeva Upadhyaya KS, Perioperative blood managementIndian J Anaesth 2014 58(5):573-80.10.4103/0019-5049.14465825535419 [Google Scholar] [CrossRef] [PubMed]
[8]. Simpson P, Perioperative blood loss and its reduction: The role of the anaesthetistBr J Anaesth 1992 69:498-507.10.1093/bja/69.5.4981467083 [Google Scholar] [CrossRef] [PubMed]
[9]. Ortmann E, Besser MW, Klein AA, Antifibrinolytic agents in current anaesthetic practiceBJA: Br J Anaesth 2013 111(4):549-63.10.1093/bja/aet15423661406 [Google Scholar] [CrossRef] [PubMed]
[10]. Dahuja A, Bhowmik S, Kaur R, Shayam R, Jindal S, Antifibrinolytic in reducing postoperative blood loss in total hip replacement and its effect on coagulation profile: A prospective randomized studyJ Orthop Allied Sci 2018 6:03-08.10.4103/joas.joas_2_18 [Google Scholar] [CrossRef]
[11]. Boenigk K, Verma K, Hoelscher C, Huncke KT, Lonner B, Errico T, The efficacy of antifibrinolytics at reducing blood loss in major spine surgery: A prospective randomized comparison of tranexamic acid, aminocaproic acid, and placebo: 6AP4-7Eur J Anaesth 2011 28(90)10.1097/00003643-201106001-00285 [Google Scholar] [CrossRef]
[12]. Lerman DM, Rapp TB, Minimizing blood loss in orthopaedic surgery the role of antifibrinolyticsBull Hosp Jt Dis 2015 73(2):83-89. [Google Scholar]
[13]. Wardrop D, Estcourt LJ, Brunskill SJ, Doree C, Trivella M, Stanworth S, Antifibrinolytics (lysine analogues) for the prevention of bleeding in patients with haematological disordersCochrane Database Syst Rev 2013 (7):CD00973310.1002/14651858.CD009733.pub2 [Google Scholar] [CrossRef]
[14]. Li G, Sun TW, Luo G, Zhang C, Efficacy of antifibrinolytic agents on surgical bleeding and transfusion requirements in spine surgery: a meta-analysisEur Spine J 2017 26(1):140-54.10.1007/s00586-016-4792-x27671279 [Google Scholar] [CrossRef] [PubMed]
[15]. Benoni G, Lethagen S, Nilsson P, Fredin H, Tranexamic acid, given at the end of the operation, does not reduce postoperative blood loss in hip arthroplastyActa Orthopaedica Scandinavica 2000 71(3):250-54.10.1080/00016470031741183410919295 [Google Scholar] [CrossRef] [PubMed]
[16]. Baradaranfar MH, Dadgarnia MH, Mahmoudi H, Behniafard N, Atighechi S, Zand V, The effect of topical tranexamic acid on bleeding reduction during functional endoscopic sinus surgeryIran J Otorhinolaryngol 2017 29(91):69-74. [Google Scholar]
[17]. Shehata A, Ibrahim MS, Abd-El-Fattah MH, Topical tranexamic acid versus hot saline for field quality during endoscopic sinus surgeryEgypt J Otolaryngol 2014 30:327-31.10.4103/1012-5574.144965 [Google Scholar] [CrossRef]
[18]. Jahanshahi J, Hashemian F, Pazira S, Bakhshaei MH, Farahani F, Abasi R, Effect of topical tranexamic acid on bleeding and quality of surgical field during functional endoscopic sinus surgery in patients with chronic rhinosinusitis: A triple blind randomized clinical trialPLoS ONE 2017 9(8):e10447710.1371/journal.pone.010447725133491 [Google Scholar] [CrossRef] [PubMed]
[19]. El Shal SM, Hasanein R, Effect of intravenous tranexamic acid and epsilon aminocaproic acid on bleeding and surgical field quality during functional endoscopic sinus surgery (FESS)Egypt J Anaesth 2015 31(1):01-07.10.1016/j.egja.2014.08.002 [Google Scholar] [CrossRef]
[20]. Chhapola S, Matta I, Short-term use of tranexamic acid to reduce blood loss in endoscopic nasal surgeriesClin Rhinol: An International Journal 2011 4:79-81.10.5005/jp-journals-10013-1078 [Google Scholar] [CrossRef]
[21]. Alimian M, Mohseni M, The effect of intravenous tranexamic acid on blood loss and surgical field quality during endoscopic sinus surgery: a placebo-controlled clinical trialJ Clin Anesth 2011 23(8):611-15.10.1016/j.jclinane.2011.03.00422137511 [Google Scholar] [CrossRef] [PubMed]
[22]. Dongare VR, Saundattikar GY, Comparison of intraoperative bleeding and surgical fields with and without tranexamic acid in Functional endoscopic sinus surgeryAnaesthesist 2018 7:233-36.10.18231/2394-4994.2018.0043 [Google Scholar] [CrossRef]
[23]. Langille MA, Chiarella A, Cote DW, Mullholand G, Sowerby LJ, Dziegielewski PT, Intravenous tranexamic acid and intraoperative visualization during functional endoscopic sinus surgery: a double-blind randomized controlled trialInt Forum Allergy Rhinol 2013 3:315-31.10.1002/alr.2110023044919 [Google Scholar] [CrossRef] [PubMed]
[24]. Abbasi HA, Behdad SD, Ayatollahi VE, Nazemian NB, Mirshamsi PB, Comparison of two doses of tranexamic acid on bleeding and surgery site quality during sinus endoscopy surgeryAdv Clin Exp Med 2012 21(6):773-80. [Google Scholar]
[25]. Powell-Tuck J, Gosling P, Lobo DN, Allison SP, Carlson GL, Gore M, British Consensus Guidelines on Intravenous Fluid Therapy for Adult Surgical Patients. Revised Recommendations from BAPEN Medical- a core group of BAPEN, the Association for Clinical Biochemistry, the Association of Surgeons of Great Britain and Ireland, the Society of Academic and Research Surgery, the Renal Association and the Intensive Care Society March 2011 [Google Scholar]
[26]. Athanasiadis T, Beule A, Embate J, Steinmeier E, Field J, Wormald PJ, Standardized video-endoscopy and surgical field grading scale for endoscopic sinus surgery: A multi-centre studyLaryngoscope 2008 118:314-19.10.1097/MLG.0b013e318157f76417989575 [Google Scholar] [CrossRef] [PubMed]
[27]. Moise A, Agachi L, Dragulin E, Mincu N, Stelea G, Tranexamic acid reduces with 50% the total nasal bleeding of patients that underwent functional endoscopic sinus surgery: 6AP6-6Eur J Anaesthesiol 2010 27(47):11510.1097/00003643-201006121-00368 [Google Scholar] [CrossRef]
[28]. Nuhi S, Tabrizi AG, Zarkhah L, Ashrafi BR, Impact of intravenous tranexamic acid on hemorrhage during endoscopic sinus surgeryIran J Otorhinolaryngol 2015 27(82):349-54. [Google Scholar]
[29]. Dunn CJ, Goa KL, Tranexamic acid: a review of its use in surgery and other indicationsDrugs 1999 57(6):1005-32.10.2165/00003495-199957060-0001710400410 [Google Scholar] [CrossRef] [PubMed]