Case Report
A 39-year-old male presented to dermatology outpatient with history of fever for 1 year, painful skin eruptions on trunk and upper limbs, oral erosions for 2 days and redness with pain and swelling in the eyes for 1 day. His fever was intermittent and high grade, for which he received ampicillin and chloroquine (dosage not known) in the past without any improvement; along with paracetamol. Four days prior to presentation, he was diagnosed with dermatophytosis; for which he was receiving 150 mg of fluconazole daily at the time of presentation. He noticed a rash of different morphology on the trunk and oral ulcerations, 2 days after starting fluconazole.
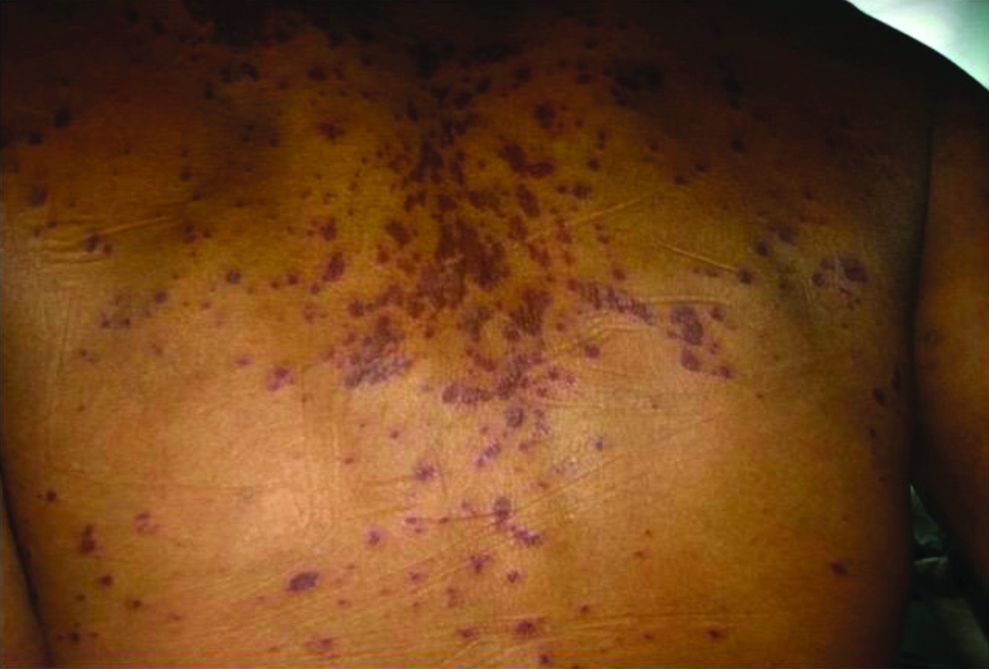
Thereafter, some of the skin eruptions became fluid-filled over face and abdomen. He had difficulty in swallowing due to the oral mucosal ulcerations. He had no past history of drug allergy. He was a driver by occupation and addicted to both tobacco and alcohol. On examination, the patient was fully conscious and oriented. His pulse rate was 100/minute regular, respiratory rate 25/minute regular. His body temperature was 102°F. There was bilateral, symmetrical, generalised lymphadenopathy. Bilateral axillary and inguinal lymph nodes were soft, 2 cm in size, discrete, mobile and non-tender. Liver and spleen were soft with smooth surface 1 cm below the costal margin. The affected body surface area involved by the skin rash was calculated to be 8% (Wallace rule of nines) [1]. Both the eyes had non-purulent bulbar conjunctival congestion, blepharitis and chemosis. Non blanchable maculopapular lesions were present on the face [Table/Fig-1], chest [Table/Fig-2] back [Table/Fig-3] and upper limbs with few blisters and peeled up skin along with erosions and haemorrhagic crusts on the buccal mucosa and the lips. Nikolsky’s sign was absent. The differential diagnosis considered was SJS, pemphigus foliaceus and Staphylococcal Scalded Skin Syndrome (SSSS). SJS was taken as the most likely diagnosis.
Non blanchable macular and papular lesions and mucosal erosions.
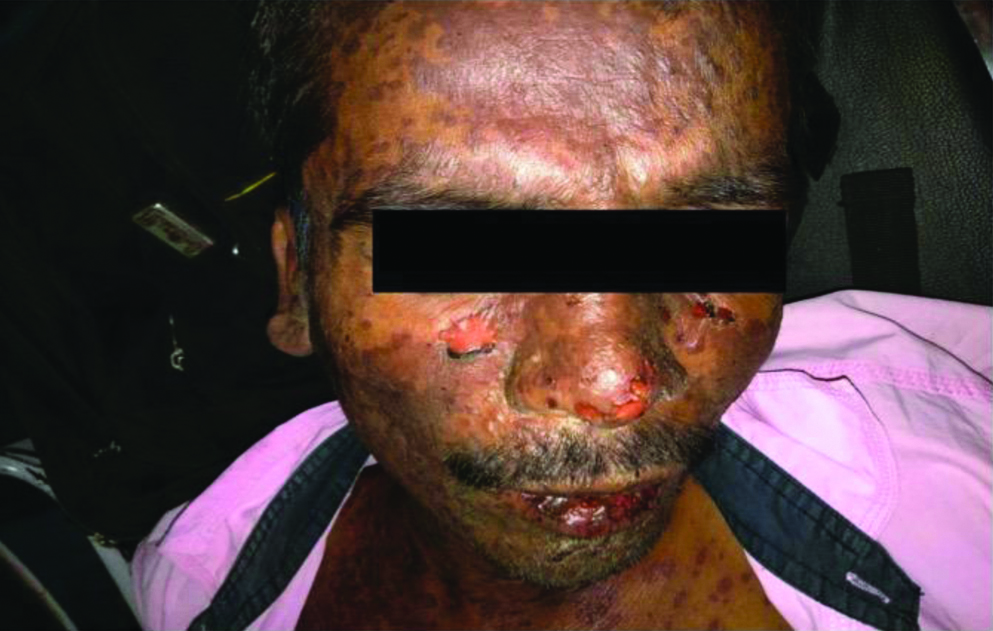
Presence of non blanchable macules and papules (few erosions and bullae can be seen).
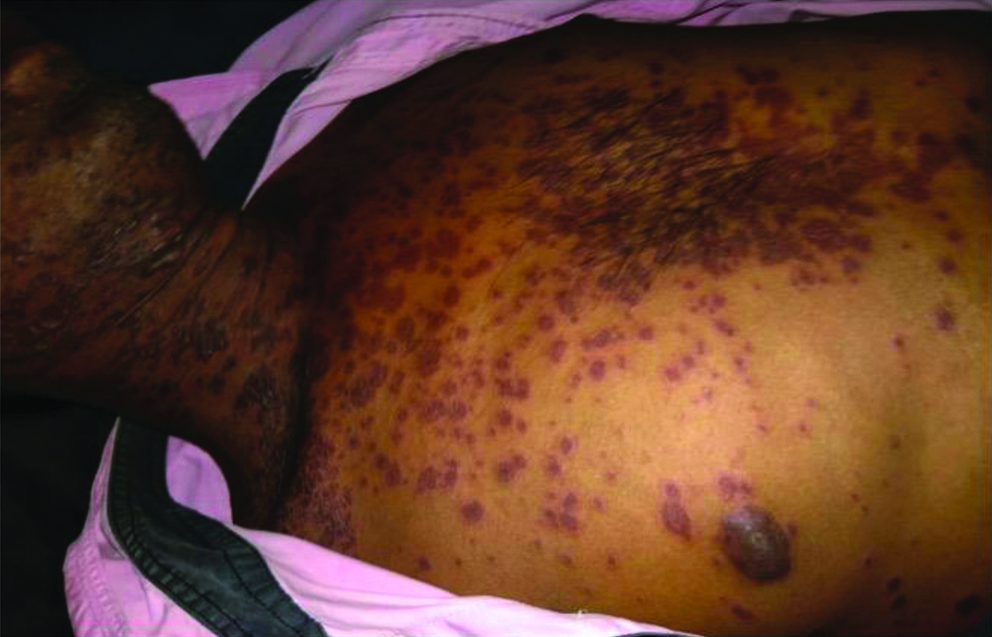
Non blanchable maculopapular lesions.
Laboratory investigations were done which revealed haemoglobin of 10.2 gm%, total leukocyte count 14,000 /cumm, polymorphs 71%, lymphocytes 26% and eosinophils 03%, C-reactive Protein (CRP) 25 mg/dL, blood urea 99 mg/dL, creatinine 1.6 mg/dL, uric acid 11.2 mg/dL, total bilirubin 0.5 mg/dL and total proteins 7.2 gm/dL. Ultrasound Abdomen confirmed hepatosplenomegaly. Hepatitis A IgM antibody, Hepatitis B surface antigen and core IgM antibody assay, hepatitis C viral RNA and hepatitis C IgM antibody assay were negative. The test for HIV1 was positive. His CD4+ T cell count was 32 cells/cumm.
The final diagnosis of SJS was made on the basis of history of fluconazole (drug intake), sudden onset of dusky generalised erythematous non blanchable rash, mucosal involvement and confirmed by skin biopsy. The skin biopsy from a blister from the back revealed necrotic keratinocytes in the epidermis and mild infiltrate of lymphocytes and few eosinophils in the dermis. The Naranjo score which is an adverse drug reaction probability scoring system was calculated to be eight in the present case.
The patient responded to withdrawal of fluconazole and conservative management. Intravenous fluids were administered to maintain the fluid and electrolyte balance untill the patient started taking soft diet orally (3 days). Eyes were cleaned with saline and antibiotic eye drops were given. Repeated Povidone Iodine (5% diluted in 100 mL of water) mouthwash was done before and after meals. As there were no raw areas on the skin, no specific treatment was given for the skin. Highly active antiretroviral therapy was started from the Anti Retroviral Treatment (ART) centre consisting of zidovudine 300 mg twice daily, lamivudine 150 mg twice daily and nevirapine 200 mg twice daily. Provocation with fluconazole was not attempted. The patient was discharged after 15 days when his eye and oral lesions healed. The patient has been following-up regularly in ART clinic and was doing well after treatment.
Discussion
An adverse cutaneous drug reaction refers to a noxious and undesirable change in the skin, mucous membranes or appendages caused by a drug [2,3]. There are different clinical and morphological presentation of cutaneous drug reaction such as anaphylaxis, urticaria, angioedema, drug induced hypersensitivity syndrome, acute generalised exanthematous pustulosis, erythema multiforme, SJS, Toxic Epidermal Necrolysis, serum sickness, exanthematous eruption, fixed drug eruption, psoriasiform drug eruption, lichen planus like lesions and acneiform eruptions [4].
SJS is an acute, rare, potentially fatal and severe mucocutaneous disorder that has an incidence of 1-6 cases per million person-years [5]. The eruption is generally a maculopapular rash with/without a bullous component. The epidermal necrosis and detachment, mucosal involvement and constitutional signs and symptoms are characteristic of this disease.
SJS and TEN are clinical variants. Previously these were referred to as Lyell Syndrome. They are differentiated on the basis of extent of body surface area involvement, prognosis and mortality. A <10% body surface area involvement, <5% mortality and good prognosis of the patient falls into the criteria of SJS. A 10-30% body surface area involvement falls into SJS/TEN overlap. Whereas >30% body surface area involvement, poor prognosis, 20-30% mortality falls into the clinical criteria of TEN [6,7]. The primary lesions of SJS are flat atypical targets with a dusky red hue. Lesions of SJS are usually isolated, however, the lesions over face and trunk can show confluence. Mucosal involvement is present and systemic symptoms are usually present; as was in this case. In contrast, lesions of TEN present with epidermal detachment, large confluent areas, ill defined plaques and flat atypical targetoid dusky red lesions. Systemic symptoms and mucosal involvement are always present [8].
The risk of SJS/TEN is increased in patients harbouring certain HLA allotypes, for example, HLA B*1502 is associated with increased risk of developing SJS after exposure to aromatic anticonvulsants [1]. It is more common in women, elderly and HIV positive individuals [7].
The triggering factors for SJS include infections, such as mycoplasma, dengue, cytomegalovirus, drugs and post vaccination [7]. Numerous medications implicated include sulphonamides and penicillin, Nonsteroidal Anti-Inflammatory Drug (NSAIDs), anticonvulsants, and allopurinol [3]. Fluconazole is a triazole antifungal agent available for oral or intravenous use in the treatment of a number of localised and disseminated mycoses, namely Candida, Coccidioidomycosis and Cryptococcus [9]. Untoward effects of the drug include nausea, vomiting, headache, diarrhoea, abdominal pain, elevated liver enzymes and skin rash [10]. Less common adverse-effects include reversible alopecia, anorexia, neurotoxicity, SJS and hepatic failure [11].
Disseminated keratinocyte apoptosis in SJS and TEN are possibly immune-mediated due to drug-specific cytotoxic CD8+ T cells and NK cells and the release of granulysin from these infiltrating cells [12].
A clonal expansion [13,14] of CD8+ cytolytic T Cells (CTL’s) by major histocompatibility Class I restricted drug presentation and also by HIV infection, as a result, a distorted expansion of a less differentiated subset of CD8+ T cells occurs. There is also a quantitative and qualitative impairment of CD4+ T cells due to HIV, leading to impaired inductive signals to CD8+ T Cells, thus affecting CD8+ T cells function. Furthermore, involvement of HIV specific CD8+ cytolytic T cells due to aberrant immune activation may also be contributing to SJS development. Thus, development of SJS due to fluconazole is more likely to occur in those patients who have HIV infection. A deficiency in cytochrome p450 and/or glutathione (more common in AIDS patients) has also been proposed to play a role in causation of SJS/TEN due to fluconazole [3].
This is a clinically SJS case who was HIV positive in which a T cell CD4 count was lower i.e., 32 cells/cumm. This case of SJS due to fluconazole was found to be suffering from HIV infection and very low CD4+ T Cell count of 32 cells/cumm. As his skin condition healed up completely before discharge from the ward except for pigmentary changes, he did not require any further intervention and highly active antiretroviral therapy consisting of zidovudine, lamivudine and nevirapine in the dosage of 300 mg twice daily, 150 mg twice daily and 200 mg twice daily respectively was started for the patient. The patient is being continuously monitored in the ART clinic. He has been given a card mentioning ‘fluconazole not to be taken’.
There are three previously reported cases of SJS due to fluconazole and four previously reported cases of TEN due to fluconazole [Table/Fig-4]. One previous case, by Gussenhoven MJ et al.,; along with the present one developed SJS to fluconazole in a HIV positive patients with a low CD 4+ T cell count. Gussenhoven MJ et al., reported a low CD4+ T cell count of 90 \cumm [15].
Previously reported cases of Steven Johnson Syndrome (SJS)/Toxic Epidermonecrolysis (TEN) due to fluconazole [3,5,6,15-18].
| Name of author | Year | Clinical diagnosis | Seropositive status | CD4+T cell count (in Cumm) |
|---|
| 1 | Gussenhoven MJ et al., [15] | 1991 | SJS | Positive | 90 |
| 2 | Azón-Masoliver A and Vilaplana J, [16] | 1993 | TEN | Positive | 33 |
| 3 | Keshari UP et al., [5] | 2014 | SJS | Negative | Not described |
| 4 | Lester LJ et al., [17] | 2008 | TEN | Positive | Not described |
| 5 | Monastirli A et al., [6] | 2008 | SJS | Negative | Not described |
| 6 | Ofoma UR and Chapnick EK, [3] | 2009 | TEN | Unknown (patient declined testing) | Not described |
| 7 | George J et al., [18] | 2012 | TEN | Positive | 625 |
Besides the case of SJS reported due to fluconazole by Gussenhover MJ et al., in a HIV positive patient, 2 other cases have been reported by Keshari UP et al., and Monastirli A et al., [5,6,15]. Monastirli A et al., reported the development of SJS in a 50-year-old caucasian women who took fluconazole for vaginal candidiasis [6]. Keshari UP et al., report a 25-year-old girl who self-administered fluconazole for vulvovaginal candidiasis [5].
Four cases of TEN have been reported previously, three of which were HIV positive, whereas in the fourth case, the patient denied testing for HIV [Table/Fig-4].
Thus an aberrant immune activation seems to be important contributory factor for SJS to develop, in patients who receive fluconazole. It thus becomes imperative that patients of SJS/TEN be investigated for their immune status, particularly if they have received fluconazole.
Conclusion
The patients having HIV and low CD4 count are likely to develop severe drug reaction in the form of SJS/TEN; so while prescribing fluconazole it will be worthwhile to be cautious in patients in whom any immunosuppressive disorder is suspected. Moreover, patients who come with SJS/TEN should be investigated for their immune status.
[1]. Moore RA, Waheed A, Burns B, Rule of Nines. [Updated 2019 May 30]In: StatPearls [Internet] 2019 Jan Treasure Island (FL)StatPearls PublishingAvailable from: https://www.ncbi.nlm.nih.gov/books/NBK513287/ [Google Scholar]
[2]. Nayak S, Acharjya B, Adverse cutaneous drug reactionIndian J Dermatol 2008 53(1):02-08.10.4103/0019-5154.3973219967009 [Google Scholar] [CrossRef] [PubMed]
[3]. Ofoma UR, Chapnick EK, Fluconazole induced toxic epidermal necrolysis: a case reportCases Journal 2009 2:907110.1186/1757-1626-2-907120062708 [Google Scholar] [CrossRef] [PubMed]
[4]. Sacchidanand S, Oberai C, Inamadar AC, IADVL Textbook of Dermatology 2015 4th edBhalani Book Depot [Google Scholar]
[5]. Keshari UP, Kumar N, Kumar R, Gari M, Fluconazole-induced Stevens-Jonson syndromeInternational Journal of Basic & Clinical Pharmacology 2014 3:566-68.10.5455/2319-2003.ijbcp20140629 [Google Scholar] [CrossRef]
[6]. Monastirli A, Pasmatzi E, Vryzaki E, Georgiou S, Tsambaos D, Fluconazole-induced Stevens-Johnson syndrome in a HIV-negative patientActa Derm Venereol 2008 88(5):521-22.10.2340/00015555-048918779900 [Google Scholar] [CrossRef] [PubMed]
[7]. Oakley AM, Krishnamurthy K, Stevens Johnson Syndrome (Toxic Epidermal Necrolysis). 2018 Dec 28StatPearls [Internet] 2019 Jan Treasure Island (FL)StatPearls PublishingAvailable from http://www.ncbi.nlm.nih.gov/books/NBK459323/PubMed PMID: 29083827 [Google Scholar]
[8]. Harr T, French LE, Toxic epidermal necrolysis and Stevens-Johnson syndromeOrphaned J Rare Dis 2010 5:39Published 2010 Dec 16.10.1186/1750-1172-5-3921162721 [Google Scholar] [CrossRef] [PubMed]
[9]. Zervos M, Meunier F, Fluconazole (Diflucan): a reviewInt J Antimicrob Agents 1993 3:147-70.10.1016/0924-8579(93)90009-T [Google Scholar] [CrossRef]
[10]. Kowalsky SF, Dixon DM, Fluconazole: a new antifungal agentClin Pharm 1991 10:179-94. [Google Scholar]
[11]. Amichai B, Grunwald MH, Adverse drug reactions of the new oral antifungal agents- terbinafine, fluconazole, and itraconazoleInt J Dermatol 1998 37:410-15.10.1046/j.1365-4362.1998.00496.x9646122 [Google Scholar] [CrossRef] [PubMed]
[12]. Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysisNat Med 2008 14:1343-50.10.1038/nm.188419029983 [Google Scholar] [CrossRef] [PubMed]
[13]. Nassif A, Bensussan A, Dorothée G, Mami-Chouaib F, Bachot N, Bagot M, Drug specific cytotoxic T-cells in the skin lesions of a patient with toxic epidermal necrolysisJ Invest Dermatol 2002 118:728-33.10.1046/j.1523-1747.2002.01622.x11918724 [Google Scholar] [CrossRef] [PubMed]
[14]. Nassif A, Bensussan A, Boumsell L, Deniaud A, Moslehi H, Wolkenstein P, Toxic epidermal necrolysis: effector cells are drug-specific cytotoxic T cellsJ Allergy Clin Immunol 2004 114:1209-15.10.1016/j.jaci.2004.07.04715536433 [Google Scholar] [CrossRef] [PubMed]
[15]. Gussenhoven MJ, Haak A, Peereboom-Wynia JD, van’t Wout JW, Stevens-Johnson syndrome after fluconazoleLancet 1991 338(8759):12010.1016/0140-6736(91)90112-3 [Google Scholar] [CrossRef]
[16]. Azón-Masoliver A, Vilaplana J, Fluconazole-induced toxic epidermal necrolysisin a patient with human immunodeficiency virus infectionDermatology 1993 187(4):268-69.10.1159/0002472618274783 [Google Scholar] [CrossRef] [PubMed]
[17]. Lester LJ, Brantley JS, Kelso RL, Kelly BC, Petitt MS, Wilkerson MG, Severe cutaneous adverse drug reaction due to fluconazoleJ Drugs Dermatol2008(11):1084-87. [Google Scholar]
[18]. George J, Sharma A, Dixit R, Chhabra N, Sharma S, Toxic epidermal necrolysis caused by fluconazole in a patient with human immunodeficiency virus infectionJ Pharmacol Pharmacother 2012 3(3):276-78.10.4103/0976-500X.9944523129968 [Google Scholar] [CrossRef] [PubMed]