Haematological abnormalities are a common finding in Systemic Lupus Erythematosus (SLE) patients. The wide spectrum includes anaemia, cytopenias, thrombosis as well as bleeding. Among the bleeding disorders, qualitative coagulation defects can be rarely encountered because of clotting factor inhibitors. More uncommon is the occurrence of quantitative clotting factor deficiencies. Here, we describe a rare cause of abdominal pain in a patient with SLE who developed perinephric and colonic sub mucosal haematomas owing to a rare association with acquired factor XIII deficiency which was diagnosed on quantitative assays and effectively treated with cryoprecipitate and fresh frozen plasma.
Case Report
A 21-year-old young lady presented to the Emergency Department with left loin pain, vomiting and loose stools for the last one day. She did not report any fever, radiation of pain, urinary symptoms, pain aggravation on food intake or any prior strenuous activity.
The patient was diagnosed with SLE 2 years ago and was on treatment with steroids, Mycophenolate Mofetil (MMF) and Hydroxychloroquine. She reported history of intermittent episodes of pain in her calf muscles in the last one year. Magnetic resonance imaging (MRI) had revealed the presence of organised haematomas in calf muscles, but the patient did not follow-up due to spontaneous resolution of symptoms.
On examination, the patient was pale, tachycardic and hypotensive. No rashes were noted. Respiratory system examination fairly ruled out pleurisy and musculoskeletal examination was normal. A non-tender firm ballotable mass was palpable in the Left lumbar region.
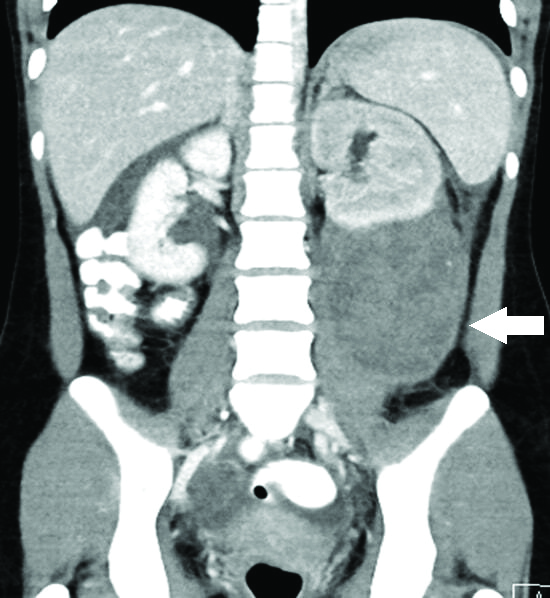
Preliminary investigations showed microcytic anaemia, (haemoglobin 6.2 g/dL), severe thrombocytopenia (22×109/L) and mild neutrophilic leucocytosis as shown in [Table/Fig-1]. Her renal function and complements were normal with slightly elevated Erythrocytic sedimentation rate (35 mm first hour). Ultrasonogram of the abdomen showed 9.4×7.4 cm heterogeneous lesion adjacent to the lower pole of the left kidney. A computed tomography revealed the presence of left perinephric haematoma with left hydroureteronephrosis [Table/Fig-2]. Initial coagulation profile revealed normal Prothrombin Time (PT), Activated Partial Thromboplastin Time (APTT) and slightly low fibrinogen levels (1.4 g/L). Direct Coombs test turned out to be positive. Von Willebrand Factor assay was normal. Antiphospholipid Antibody (APLA) work up was done in which anti beta2 glycoprotein turned out to be negative, with Lupus anticoagulant ratio of 1.6.
Preliminary investigations showing microcytic anaemia, severe thrombocytopenia and mild neutrophilic leucocytosis.
| Day 1 | Day 5 | Day 7 | Day 11 | Standard value |
|---|
| Haemoglobin g/dL | 6.2 | 8.4 | 9.9 | 11.9 | 12-15 |
| Mean corpuscular volume fL | 73.2 | | | | 80-100 |
| White blood counts ×10^3/uL | 15 | 10.6 | | 9.2 | 4-10 |
| Platelets x10^3/uL | 22000 | 42000 | 83000 | 110 | 150-400 |
| Prothrombin time (seconds)/INR | 13.3/1.2 | 13.9/1.33 | | 13.1/1.25 | 9-12 |
| APTT (seconds) | 29.1 | 22.8 | | 28.2 | 25-34 |
| Serum fibrinogen levels mg/dL | 76 | 140 | | 342 | 200-400 |
| C3 c level/C4 mg/dL | 83/21 | | | | 90-18010-40 |
| ESR mm 1st hour | 35 | 30 | | 17 | 4-12 mm |
| HsCRP mg/dL | 0.30 | | | | <0.5 mg/dL |
| Procalcitonin ng/mL | 0.2 | | | | <0.5 |
CT abdomen showing perinephric haematoma over lower pole of left kidney with ureteric compression.
The differential diagnoses considered were acute SLE flare/bleeding due to thrombocytopenia/coagulation defects and MMF induced haematological crisis. However, complement activity, disease markers were normal and the patient was on irregular treatment with MMF. Since initial PT, APTT levels were normal, possible factor XIII deficiency was considered and she was evaluated accordingly.
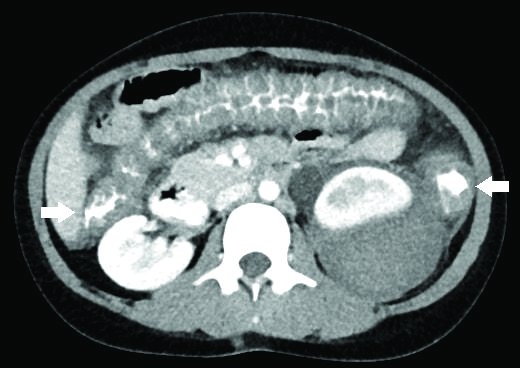
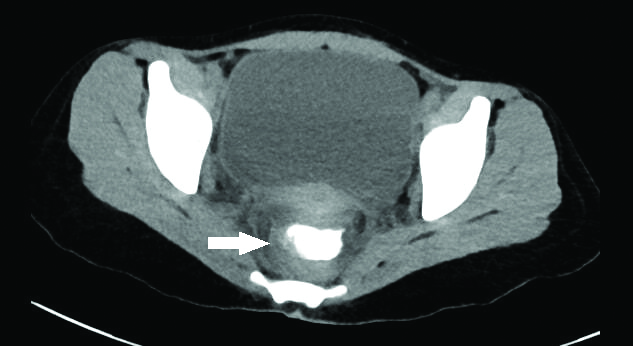
The patient was stabilised with intravenous fluids namely Normal Saline, low dose methyl prednisolone 250 mg over 3 days and 2 units packed red blood cells transfusion. There was recurrence of vomiting and loose stools during initiation of enteral feeds and a repeat CT abdomen revealed new onset transmural haematomas in small bowel loops, distal ascending colon, transverse colon, descending colon and rectum [Table/Fig-3,4]. The coagulation profile results reaffirmed normal APTT levels; repeat fibrinogen levels were normal (3.4 g/L) and platelet counts were improving (120×109/L). On performing a clot solubility test, the clot completely dissolved in 5M urea solution and on mixing studies, the clot dissolved partially. Since the results were equivocal, it was not possible to differentiate a hereditary factor XIII deficiency from a factor XIII inhibitor in this case. Subsequently, a factor XIII assay was done which turned out to be <1% thus confirming severe deficiency. Patient was transfused with a total of 18 unit’s cryoprecipitate (270 mL) and 10 units of fresh frozen plasma (2400 mL total) during her entire hospital stay following which she showed steady improvement.
CT abdomen depicting transmural haematoma of distal ascending, transverse and descending colon.
CT abdomen showing transmural haematoma involving rectum.
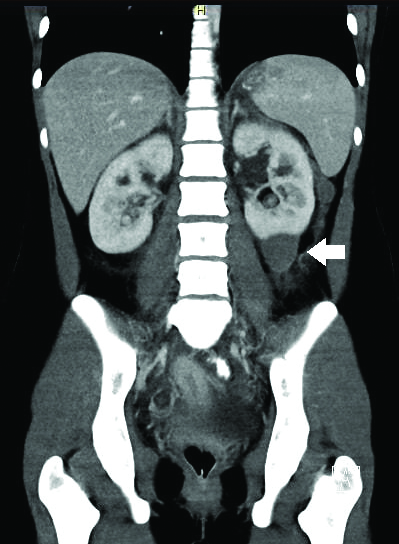
A follow-up CT abdomen a month later showed resolving left perinephric haematomas with resolution of colonic and rectal haematomas. The patient is currently doing well on 5 mg once daily prednisolone and azathioprine [Table/Fig-5].
CT abdomen revealing resolving haematoma over the lower pole of left kidney.
Discussion
SLE is a challenging disease to treat. In the current era, diagnosing SLE has become common due to increasing awareness, knowledge of its varied presentations and available investigations. However, the course of the disease poses a constant challenge to the treating physician owing to the myriad of systemic manifestations and complications.
Acute abdomen in SLE patients is a challenging diagnostic and therapeutic problem. Most patients are on steroids and/or immunosuppressive agents which mask the physical findings, thus leading to a delayed definitive diagnosis. The reported incidence of abdominal pain in SLE varies from 8 to 40% [1,2]. Assuming that all are due to vasculitis may lead to diagnostic errors. In an observation of 36 active SLE patients presenting with acute abdomen by Medina F et al., 19 had vasculitis, 3 had Anti Phospho Lipid Antibody (APLA) related intra-abdominal thrombosis and the rest were non-SLE related [3]. Therefore, it is imperative to carefully evaluate such a patient based on their clinical, imaging and immunological profile.
Haematological abnormalities are common findings in patients with SLE. The prevalence is described as 47% in most studies [4]. In a study by Sasidharan PK et al., haematological manifestations were found to be the most common initial presentation of SLE and it was the presenting manifestation in 82% of the patients. The haematological spectrum encompasses anaemia, leucopenia, thrombocytopenia, autoimmune haemolytic anaemia, thrombotic thrombocytopenic purpura, myelofibrosis-hypoplastic anaemia and thrombotic manifestations if associated with APLA [5]. The index patient too had leucopenias along with cutaneous manifestations in her initial presentation leading to her diagnosis.
It is imperative to distinguish haematological abnormalities arising due to disease activity from those due to drug effects. The index patient had normal complement activity and disease markers making acute SLE flare unlikely. The patient was on irregular treatment with MMF, making drug related side effect a remote consideration. Bleeding despite normalisation of platelet count, fibrinogen levels and normal PT, APTT prompted us to investigate us for rare clotting factor abnormalities, such as Factor XIII defects. Coagulation defects in SLE could be due to inhibitors more commonly [6], and due to factor deficiencies rarely [7-9].
Factor XIII is needed to stabilise the fibrin clots and it’s deficiency could be genetically acquired as Autosomal recessive disorder or could be due to acquired causes [7]. Hereditary deficiency is attributed to the F13A1 gene located on the short arm of chromosome 6 (6p24.2- p23). Acquired factor XIII deficiency occurs much less often, and usually affects middle-aged or elderly individuals [10]. It can be associated with conditions such as severe liver disease, chronic kidney failure, inflammatory bowel disease, Henoch-Schonlein purpura, autoimmune disorders such as SLE or scleroderma and certain cancers such as myeloid forms of leukaemia. It has also been linked to the use of certain medications including isoniazid, penicillin, and phenytoin.
Individuals with factor XIII deficiency form blood clots per se, but the clots are unstable and often break down, resulting in prolonged, uncontrolled mild to fatal bleeding episodes. The most common bleeding symptoms are subcutaneous bleeding (57%), delayed umbilical cord bleeding (56%), muscle haematoma (49%), haemorrhage after surgery (40%), haemarthrosis (36%) and intracerebral bleeding (34%) [11,12]. However, the index patient’s presentation was unusual with perinephric, colonic submucosal and transmural haematomas.
Factor XIII deficiency is screened by clot solubility test and confirmed by quantitative assays [13]. It is treated by factor XIII concentrates, fresh frozen plasma or cryoprecipitate. When inhibitors develop in individuals with factor XIII deficiency, additional therapy in the form of immunosuppressive agents would be required.
On review of literature, there is a reported Lupus patient with associated factor 13 deficiency who developed intracerebral haematoma [9]. There are case reports of subcutaneous and intramuscular haematomas due to factor XIII deficiency following an invasive procedure in a patient with SLE [14]. In another instance a 70-year-old man had developed spontaneous left gluteal haematoma owing to factor XIII deficiency [15]. Occurrence of gastrointestinal haematomas as in the index patient seems to be extremely rare.
Conclusion
It is fruitful to think beyond lupus while approaching the presenting complaints even in a diagnosed patient of SLE. Attributing all the features to the disease per se might be misleading. A high index of suspicion for rarer clotting factor deficiencies is required while evaluating the bleeding manifestations with a normal coagulation profile. There exists a lacuna in ascertaining the fact as to why in an autoimmune disease, there is associated clotting factor deficiency, unlike the expected scenario of clotting factor inhibitors. Only continued medical research can unravel such mysteries.
Author Declaration:
Financial or Other Competing Interests: No
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Apr 02, 2019
Manual Googling: Oct 21, 2019
iThenticate Software: Nov 20, 2019 (20%)
[1]. Sultan SM, Ioannou Y, Isenberg DA, A review of gastrointestinal manifestations of systemic lupus erythematosusRheumatology 1999 38(10):917-32.10.1093/rheumatology/38.10.91710534541 [Google Scholar] [CrossRef] [PubMed]
[2]. Zizic TM, Classen JN, Stevens MB, Acute abdominal complications of systemic lupus erythematosus and polyarteritis nodosaThe American Journal of Medicine 1982 73(4):525-31.10.1016/0002-9343(82)90331-X [Google Scholar] [CrossRef]
[3]. Medina F, Ayala A, Jara LJ, Becerra M, Miranda JM, Fraga A, Acute abdomen in systemic lupus erythematosus: The importance of early laparotomyThe American Journal of Medicine 1997 103(2):100-05.10.1016/S0002-9343(97)80020-4 [Google Scholar] [CrossRef]
[4]. Bennett JC, Claybrook J, Kinsey H, Holley HL, The clinical manifestations of systemic lupus erythematosus. A study of forty-five patientsJournal of Chronic Diseases 1961 13(5):411-25.10.1016/0021-9681(61)90027-3 [Google Scholar] [CrossRef]
[5]. Sasidharan P, Bindya M, Sajeeth Kumar K, Hematological manifestations of SLE at initial presentation: Is it underestimated?ISRN Hematology 2012 :1-5.10.5402/2012/96187222830038 [Google Scholar] [CrossRef] [PubMed]
[6]. Bashal F, Hematological disorders in patients with systemic lupus erythematosusThe Open Rheumatology Journal 2013 7(1):87-95.10.2174/187431290130701008724198852 [Google Scholar] [CrossRef] [PubMed]
[7]. Chuliber F, Schutz NP, Otero V, Barrera L, Altuna D, Fantl DB, Acquired Factor XIII deficiency in the inpatient setting: Clinical suspicion, impact and treatment: Retrospective case seriesBlood 2016 128(22):141510.1182/blood.V128.22.1415.1415 [Google Scholar] [CrossRef]
[8]. Sawlani KK, Chaudhary SC, Roy A, Tripathi AK, Factor XIII deficiency presenting with intracerebral bleedBMJ Case Rep 2013 2013:bcr201200730310.1136/bcr-2012-00730323314446 [Google Scholar] [CrossRef] [PubMed]
[9]. Dabadghao VS, Singh VB, Meena BL, Khichar S, Systemic lupus erythematosus with intracerebral hematoma due to decreased factor XIII activity: A rare associationJ Mahatma Gandhi Inst Med Sci 2013 18:129-31.10.4103/0971-9903.117792 [Google Scholar] [CrossRef]
[10]. NORD (National Organization for Rare Disorders). (2019). Factor XIII Deficiency- NORD (National Organization for Rare Disorders). [online] Available at: https://rarediseases.org/rare-diseases/factor-xiii-deficiency [Accessed 6 Oct. 2019] [Google Scholar]
[11]. Acharya SS, Coughlin A, DiMichele DM, The North American Rare Bleeding Disorder Study Group. Rare Bleeding Disorder Registry: deficiencies of factors II, V, VII, X, XIII, fibrinogen and dysfibrinogenemiasJ Thromb Haemost 2004 2:248-56.10.1111/j.1538-7836.2003.t01-1-00553.x14995986 [Google Scholar] [CrossRef] [PubMed]
[12]. Ivaskevicius V, Seitz R, Kohler HP, Schroeder V, Muszbek L, Ariens RA, International registry on factor XIII deficiency: a basis formed mostly on European dataThromb Haemost 2007 97(6):914-21.10.1160/TH07-01-003417549292 [Google Scholar] [CrossRef] [PubMed]
[13]. Kohler HP, Ichinose A, Seitz R, Ariens RAS, Muszbek L, On behalf of the Factor XIII and Fibrinogen SSC Subcommittee of the ISTH. Diagnosis and classification of factor XIII deficienciesJ Thromb Haemost 2011 9:1404-06.10.1111/j.1538-7836.2011.04315.x22946956 [Google Scholar] [CrossRef] [PubMed]
[14]. Rabik CA, Atkinson MA, Sule S, Strouse JJ, Treatment of an acquired Factor XIII inhibitor in an adolescent with systemic lupus erythematosus and renal failureTransfusion 2017 57(9):2159-63.10.1111/trf.1418528707410 [Google Scholar] [CrossRef] [PubMed]
[15]. Fogarty H, Byrne M, O’Connell NM, Ryan K, White B, O’Donnell JS, Acquired factor Xiii deficiency: an uncommon but easily missed cause of severe bleedingIr Med J 2018 111(5):757 [Google Scholar]