Quality control in blood banking ensures the availability of blood components of high standards to the potential recipients at right time and with maximum efficacy and minimal risk [4]. It involves various aspects of blood banking operations like quality control of reagents, equipment calibrations, clerical checks, temperature monitoring of refrigerators, volume of blood products and quality indicators of various components [5].
Quality control of whole blood and PRBCs involves estimation of volume and haematocrit [6]. Quality of platelet concentrate is assessed by parameters like swirling movement, volume, platelet yield, and RBC and WBC counts per unit and pH changes [7]. As this is imperative for the preparation of components of optimal quality, regular periodic assessment of quality control parameters as per national guidelines is of utmost importance.
Same sample was also used for the study of quality control of Fresh Frozen Plasma using Fibrinogen and Factor VIII as a parameter for the same period of time [8].
Materials and Methods
The retrospective data of routine monthly analysis of whole blood and blood components was collected from archives of blood bank from the period of 1st January 2017 to 31st December 2017 in Adesh Institute of Medical Sciences and Research, Bathinda, Punjab, India. Ethical approval from institution was taken with number AU/EC/FM/151/2018.
Data was collected for the above mentioned period in which blood was collected from 3476 healthy donors (more than 45 kg) in sterile single, double or triple blood bags with anticoagulant Citrate Phosphate Dextrose Adenine 1 (CPDA 1) after taking written consent. Out of these 3476 units, 1321 units were utilised as whole blood (350 mL of whole blood from donors weighing 45-60 kg) and 2155 units collected in double or triple bags (450 mL blood) from healthy donors weighing more than 60 kg were processed for component preparation in a refrigerated centrifuge (Cryofuge 5500i) by Heraeus.
After 2-4 hours of holding time, units were centrifuged at 3800 (4400xg) rotations per minute at 4°C for 9 minutes for separation into PRBCs and FFP. For separation of whole blood into PRBCs, FFP and platelet concentrate units were centrifuged at 2 spin centrifugation at 1500 rpm for 9 minutes at 22°C followed by 2500 (4400 x g) rpm for 15 minutes at 22°C [8].
Samples of 3.6% of total whole blood collection (48/1321), 2.3% of PRBCs (50/2155), and 9.3% of platelet concentrate (42/451) was prepared and were tested for haematological parameters for quality control as per standard guidelines given in ‘Technical Manual of Transfusion Medicine’, by Directorate General of Health Services Ministry of Health and Family Welfare, India [7].
Blood units or components which were showing gross haemolysis or discoloration were not included for quality analysis.
Whole blood and PRBCs samples were tested for total volume and hematocrit.
Volume was calculated as per formulas:
Whole blood: 1 gm=1.05 mL
PRBCs: 1 gm =1.09 mL
Platelet concentrate: 1 gm=1.035 mL
Weight was calculated as: weight of filled bag-weight of empty bag.
For measurement of weight, standard calibrated weighing scales were used.
Platelet concentrate was tested for total volume, swirling movements, platelet yield and WBC counts per unit.
Swirling movement in platelet was assessed visually after a brief squeezing along the sides of the bag against the light and documented as “present” or “absent” [2].
Hematocrit, platelet and WBC counts were done in automated hematology analyser SYSMEX XS-800i.
Statistical Analysis
Statistical analysis in terms of mean, range and standard deviation was done in SPSS software version: 12.
Results
A total of 3476 units of blood were collected from healthy screened donors, out of which 1321 were utilised as whole blood and 2155 units were separated into components (FFP and PRBCs; FFP, PRBCs and PC).
Whole Blood
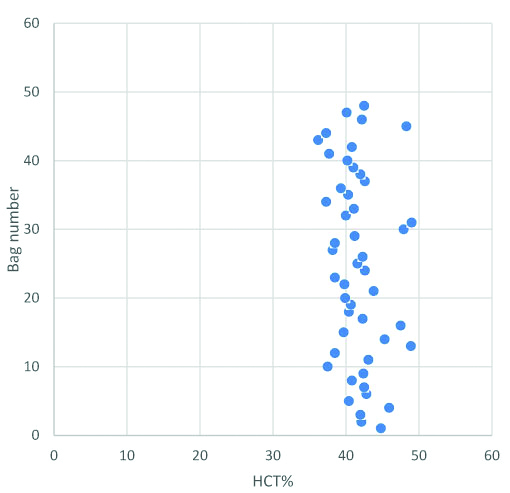
Samples of 3.6% of total whole blood collection (48/1321) were analysed for quality control of haematological parameters [Table/Fig-1]. Mean volume was 359 mL with range of 345-375 mL. Mean hematocrit was 41.7% with range of 36.2-49.0% [Table/Fig-2].
Quality control results of whole blood and PRBCs.
| Parameter | Whole blood | Packed red blood cells |
|---|
| Recommended [1,7] | Mean | Range | Concordance | Recommended [1,7] | Mean | Range | Concordance |
|---|
| Volume (in mL) | 350±10% | 359 | 345-375 | 100% | 280±60 | 310 | 270-390 | 100% |
| Hematocrit | >30% | 41.7% | 36.2-49.0% | 100% | >55% | 69.5% | 56.3%-80.9% | 100% |
Hematocrit in whole blood units: mean hematocrit was 41.7% with range of 36.2-49.0%.
Packed Red Blood Cells
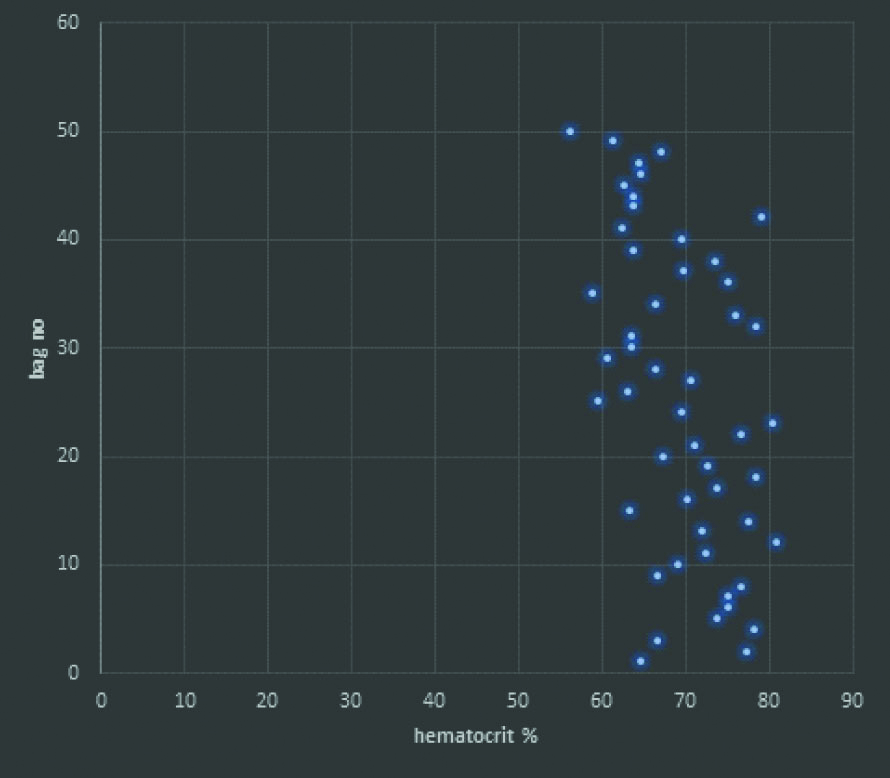
Total 50 out of 2155 (2.3%) units were tested for volume and hematocrit [Table/Fig-1]. Mean volume was 310 mL with range of 270-390 mL. Mean hematocrit was 69.5% with range of 56.3-80.9% [Table/Fig-3].
Hematocrit in packed RBC units: Mean hematocrit was 69.5% with range of 56.3-80.9%.
Platelet Concentrate
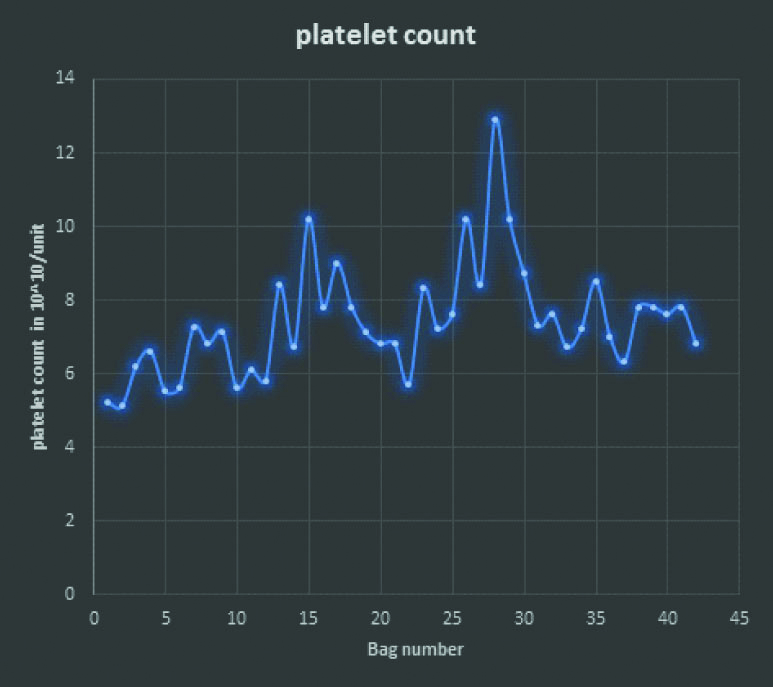
Total 42 out of 451 (9.3%) units were tested for volume, platelet count, and RBC and WBC contamination [Table/Fig-4]. Mean volume was 63.7 mL with range of 50-70 mL. Mean WBC contamination was 1.2×108/unit with a range of 0.17-5.2×108/unit. Mean RBC contamination was 0.081×1012/liter with a range of 0.02- 0.14x1012/liter [Table/Fig-4]. Mean platelet count was 7.4×1010/unit with a range of 5.1-12.9×1010/unit [Table/Fig-5].
Quality control results of platelet concentrate.
| Parameter | Quality requirement [1,7] | Mean±SD (mL) | Range | Concordance |
|---|
| Volume | 50-70 mL/bag | 63.7±4.56 | 50-70 mL | 100% |
| Inspection | Swirling movement of platelets | Present in all units |
| Platelet count | >5.5×1010/bag in 75% of bags | 7.4±1.53×1010/unit | 5.1-12.9×1010/unit | 95% |
| WBC contamination | 5.5×107-5×108 in 450 mL bag. | 1.20±1.37×108/unit | 0.17-5.2×108/unit | 97.6% |
| RBC Contamination | <0.1×1012/liter | 0.081±0.05×1012/liter | 0.02-0.14×1012/liter | 95.2% |
Platelet count per unit of platelet concentrate: Mean platelet count was 7.4 x1010/unit with range of 5.1- 12.9 x1010/unit.
Discussion
Blood banks have the dual responsibility of providing safe blood/components with maximum efficacy to the recipients as well as maintaining adequate stock and blood supply [9] “Zero risk blood supply” is the ultimate manufacturing goal of transfusion medicine [10].
Internal Quality Control is the integral part of quality assurance in all laboratory services. It is the pre-defined set of procedures that are done for continuous assessment of routine work, so as to assess the performance standards [4]. Maintaining quality control standards in blood banks help in decreasing the number of adverse blood reactions. Regular periodic quality analysis of blood components is designed to monitor variations in manufacturing processes, product quality and ensure that manufacturing steps meet defined criteria for acceptance [11].
In the present study, the overall quality control audit met the standards established within permissible limits as per national guidelines. The recommended volume for whole blood was 350±10 mL with haematocrit of >30%, and recommended volume of PRBCs was 280±60 mL with haematocrit of >55% as per standard guidelines [7]. In present study, the mean volume of whole blood units was 359 mL with a range of 345-375 mL and the mean volume of PRBCs was 310 mL with a range of 270-390 mL. The mean haematocrit of whole blood units was 41.7% with a range of 36.2-49%, whereas mean haematocrit of PRBCs was 69.5% with a range of 56.3 to 80.9%. All the whole blood and PRBCs units checked had volume and haematocrit well within standard criteria thus establishing the quality of our blood bank. Results similar to this study have been observed in study done by Upadhyay S et al., in which mean volume of whole blood units was 410±8.1 mL with a range of 391-522 mL and haematocrit of whole blood units was 43.7±3.2% with range of 38-52.5% [5]. Mean volume of PRBCs units was 285±24.3 mL with a range of 198-350 mL and haematocrit of 54±4.2% with a range of 41-69%.
Quality control parameters of platelets were also within normal range as per standard guidelines. Similar results were observed in various other studies done by Raturi M et al., Singh RP et al., Fijnheer R et al., Hirosue A et al., [Table/Fig-6] [2,6,12,13]. Raveendran R. et al., also studied the various haematological parameters of platelet concentrate. Volume and WBC contamination in their study corroborated with the present study, however, their mean platelet count was more than this study, both meeting normal standard criteria [14]. As patients with thrombocytopenia need transfusion of platelet concentrates for prevention/ stoppage of bleeding, platelet transfusion should provide good quality for adequate benefit.
Results seen in various studies in comparison to present study [2,6,12,13].
| Study | Mean volume (mL) | Mean platelet count (1010/unit) | Mean WBC count (107/unit) |
|---|
| Fijnheer R et al., [12] | 67.8±4 | 7.0±1.0 | 1.9±1.5 |
| Raturi M et al., [2] | 58.4±9.5 | 5.9±1.28 | 1.5±1.2 |
| Hirosue A et al., [13] | 41±2 | 7.62±1.8 | 3.0±1.0 |
| Singh RP et al., [6] | 62.3±22.68 | 7.6±2.97 | 4.05±0.48 |
| Present study | 63.7±4.56 | 7.4±1.53 | 1.20±1.37 |
Thus the quality parameters of platelets in our blood bank were in concordance with the above mentioned studies and with standard guidelines, thus establishing their quality.
Future Recommendations
Quality assessment should be a standard protocol for all blood banks and proper quality analysis records should be maintained for keeping the quality of blood transfusion practices up to the mark. However, more studies with larger sample size and inclusion of more quality indicator parameters for blood banking as a whole are warranted to uplift the blood transfusion practices.
Limitation
Ours being a relatively small set up, limited samples were available for quality analysis.
Conclusion
The quality of whole blood, PRBCs and platelet concentrate in our blood bank meet the standard national criteria. Safe blood transfusion is universal human right and should be made available through proper quality management for all processes in blood collection, preparation of components and issuing to the recipients. Quality indicators should be well-defined, regularly monitored and properly documented. Regular updation and adherence to standard operating procedures is of utmost importance. Participation in donor and recipient hemovigilance programme will also establish good transfusion practices.