Introduction
PJP was previously called as PCP, it is one of the most common opportunistic fungal infection in immuno-compromised conditions such as haematological malignancy, congenital immunodeficiency, organ transplantation, immunosuppressive therapy, under medication and predominantly in HIV [1]. Patients presenting with clinical symptoms are fever, non productive cough, chills, weight loss, dyspnoea, shortness of breath and respiratory failures may also occur in severe cases [2]. Person to person PJP transmission typically takes place via air borne route. If healthy individuals are infected, asymptomatic lung colonisation can occur in these people and act as asymptomatic carriers. These people can spread infection to immuno-compromised individuals; it may cause severe respiratory infection particularly in AIDS patients (AIDS associated Pneumonia). Still, Pneumocystis is a major opportunistic atypical fungus causing infection to immuno-compromised individuals [3]. Occurrence of severe Pneumocystis infection may lead to morbidity and mortality in healthy as well as immuno-compromised individuals. But availability of specific chemoprophylaxis, effective combination Highly Active Anti-Retro Viral Therapy (HAART) decreases opportunistic infections in HIV patients. Extensive prophylaxis can diminish the disease burden in this group of population [4-6].
MICROBIOLOGY
Nomenclature and Controversy on Taxonomy of Pneumocystis jirovecii
In the year 1906, Carlos Chagas identified Pneumocystis for the first time and placed them under the group of protozoan. Again in 1988, they were reclassified and considered as an ascomycetous group of fungus [6-8]. Life cycle of Pneumocystis have similar characters of both protozoan and fungus. Researchers conducted phylogenetic analysis of small subunit ribosomal RNAs and compared lineages of plant, animal, fungi and protozoa [9,10]. Finally, researchers concluded that Pneumocystis comes under the fungal group based on the characters of cell wall composition, enzyme structure and sequencing genes. Species of Pneumocystis are Pneumocystis carinii and Pneumocystis jirovecii which infect rats and humans respectively.
The scientist Otto jirovec identified the organism in humans and gave the name Pneumocystis jirovecii instead of P. carinii [7-10].
EPIDEMIOLOGY
First Case Report of PJP
First clinical case of Pneumocystis was recognised during World War II in Europe among premature and malnourished children. Later, cases were reported in children in Iranian orphanages. In 1981, Pneumocystis infection was reported in homosexuals and intravenous drug users [11,12].
Risk Factors of PJP
Major risk factors for acquiring disease of PJP include HIV, solid organ transplantation, malignancy, congenital immuno-deficiency and immuno-suppressive therapy. In non-infected HIV individuals, immuno-suppressive therapy with glucocorticoids was the major risk factor for acquiring PJP [3,6,13,14] [Table/Fig-1].
Pneumocystis infection varies based on the diagnosis and disease condition.
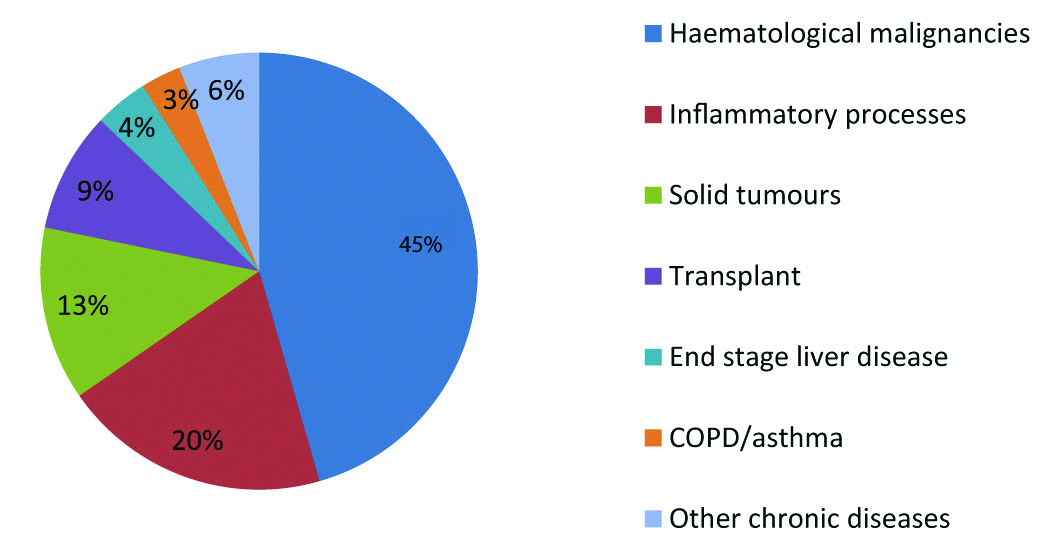
HIV Epidemic Related to PJP
HIV outbreak occurred in 1980; studies focused on illness of disease and found PJP as the important risk factor. Thereafter, PJP turned from rare disease to common pneumonia. Almost 75% of the HIV patients acquired Pneumocystis infection during their life time. Disease rate decreased from 1989, due to beginning of prophylaxis and anti-retroviral therapy. The rate of infection decreased slowly in developed countries and also in United States. Recurrence of PJP can occur more in patients with HIV infection than in non-HIV patients. Still, it is a common infection in immuno-compromised individuals especially in HIV patients and also other conditions that weaken the immune system [4,15].
In United States and Canada, PCP was the common most opportunistic infection for the period of 2008-2010 [16]. In US, no national surveillance programme was conducted on PJP. That’s the reason why exact number of cases was not reported from U.S. [17]. Cohort study was conducted among 8500 HIV infected individuals in Europe. This study reported decrease in incidence of PCP before cART 4.9 cases per 100 person-years and after cART 0.3 cases per 100 person-years [18]. During 1980s, 0-11% of Pneumocystis infections were reported in AIDS patients. Trend of Pneumocystis infection occurred in AIDS patients were raised in Africa. Higher rate of infection were reported in the year of 1995 & 2002 in Zimbabwe 33% and Zambia 22% [19-21].
Non-HIV Epidemic Related to PJP
Pneumocystis infection occurred in non-HIV individuals who are Immuno-suppressed.
Indian Perspective: Singh YN et al., first reported three cases of Pneumocystis infection with AIDS disease in 1993 [Table/Fig-2] [22-35].
Various PJP reports in India for past 19 years [22-35].
| Author | Year published | State | PJP |
|---|
| Singh YN et al., [22] | 1993 | Delhi | First reported three cases of Pneumocystis infection with AIDS disease. |
| Mirdha BR et al., [23] | 2000 | New Delhi | Reported 4 cases of Pneumocystis in 53 AIDS patients. |
| Merchant RH et al., [24] | 2001 | Mumbai, Maharashtra | Reported 3.88% of infection in under the age of 5 years. |
| Kumarasamy N et al., [25] | 2005 | Chennai, Tamil Nadu | Reported 0.7 to 7% cases in India. |
| Rajagopalan N et al., [26] | 2009 | Bangalore, Karnataka | Reported 5% cases. |
| Shahapur PR et al., [27] | 2014 | Bijapur, Karnataka | Reported 1.81% cases. |
| Chawla K et al., [28] | 2011 | Manipal, Karnataka | Reported 20% Pneumocystis cases in HIV patients. |
| Ramesh K et al., [29] | 2015 | Bellary, Karnataka | Reported 16% of Pneumocystis cases with AIDS. |
| Jairam A et al., [30] | 2014 | Delhi, India | Reported outbreak of PJP infection in renal transplant recipients (RTRs). |
| Kaur R et al., [31] | 2016 | New Delhi, India | Reported 27.2% of cases. |
| Deodhar D et al., [32] | 2018 | Ludhiana, Punjab. | Reported 30.9% of cases. |
| Jeswani J et al., [33] | 2018 | Jaipur, Rajasthan | Reported Pneumocystis infection in non-HIV group individuals, included renal transplant patients, malignancies, chronic lung disease and connective tissue disorder. |
| Shilpa et al., [34] | 2018 | Raichur, Karnataka | Reported 16% of cases. |
| Singh Y et al., [35] | 2019 | New Delhi, India | Reported 22.4% cases of Pneumocystis in HIV patients. |
In Indian tertiary care set-up, very less number of studies were reported on nosocomial PJP. In 2014, PJP outbreak was reported at a tertiary care hospital [30].
Mortality
Kumarasamy N et al., reported, 22% of deaths in AIDS patients with infection of Pneumocystis in the year of 1996 to 2008 in Chennai (South India) [36]. A study from Mumbai, reported 15.8% deaths occurred in HIV patients, in the year of 2000-2003 [37]. Rajagopalan N et al., reported 2% of death cases in Karnataka, in AIDS patients with Pneumocystis (2004-2006) [26]. Deodhar D et al., reported 46.1% of death cases in HIV group of patients and 34.6% in non-HIV group patients in the year of 2009-2014 [32]. Jeswani J et al., reported 4 deaths among 15 cases of renal transplant patients in Jaipur in the year of 2013- 2018 [33].
Present Situation
Most of the studies were conducted broadly on opportunistic infections in HIV disease. Very few studies are available on Pneumocystis jirovecii; their detection, genotypes, mutations and drug resistance from Andhra Pradesh and India. Future research will be needed on PJP infection to overcome disease burden.
Pathophysiology
Pneumocystis jirovecii is a main pathogen causing pneumonia in people with immuno-deficiency state. Basic mechanism of pathogenesis causing pneumonia is not completely understood. Pneumocystis reaches the alveoli, first interact with cells and other components. The primary binding is with type 1 alveolar epithelium, fungus transition occurs from trophic form to cystic form. This binding does not damage the alveoli but awake the host inflammatory response. Hypoxia and impaired gas exchange leads to respiratory failure. Pneumocystis is an alveolar pathogen but sometimes in immuno-compromised individuals, it acts as a disseminated form. Extra pulmonary manifestations like hepatosplenomegaly, thyroid, ear, ocular and skin lesion were shown by Pneumocystis infected immuno-compromised patients [38].
Transmission
PJP is a communicable disease. Person to person transmission occur through air borne route. If healthy people acquire infection of Pneumocystis, organism will be found in their lungs but does not show any signs and symptoms. These people can act as carriers and infection transmits to other people who are immuno-compromised or have lower immunity condition. Majority of the infections and outbreaks were reported in hospital settings. Recurrent pneumonia occurred in same persons in different episodes by involvement of genotype. PJP is easily spread in people who are defective of both cellular and humoral immunity [4].
Clinical Features
Non-specific physical findings were observed in patients with PJP. Common symptoms are fever, cough, difficulty breathing, chest pain, chills and fatigue. Mild fever was observed in people with HIV infection after several weeks of PJP infection. Almost 7% of the people are asymptomatic. Pulmonary auscultation is common; when abnormal, the most common finding is inspiratory crackles. Extra pulmonary manifestations like thyroiditis, retinitis, bone lesions, pneumocystosis of brain, liver, spleen and kidney were not common in PJP patients but these are common among patient group including advanced AIDS condition, extremely immuno-compromised and treatment taking with aerosolized Pentamidine [39].
Differential Diagnosis
Differential diagnosis facilitates proper testing to rule out possibilities and prove an absolute diagnosis. Pulmonary complications are developed with atypical presentation and PJP cannot be ruled out. A confident diagnosis requires a grouping of clinical, radiological, and laboratory investigations for giving proper treatment.
Tuberculosis: Opportunistic bacterial and fungal diseases include tuberculosis and PJP may cause illness in patients with HIV [40].
Acute Respiratory Distress Syndrome (ARDS): HIV patients with PJP are associated with a high death rate, which increases significantly with the need for mechanical ventilation after presenting with respiratory distress and severe hypoxemia [41].
Mycoplasma infections: In Pneumonia cases, presence of P. jirovecii and M. pneumoniae and in the absence of definitive diagnoses, monitoring the treatment response is vital mainly when first line antibiotic predilection is β-lactams or Cephalosporins [42,43].
Pulmonary embolism: Symptoms presented almost same as that of PJP. One of the case reports concluded the final diagnosis with chest radiograph and CT scan [44].
Mycobacterium avium Complex (MAC) Infection [40]. A Case report concluded PJP can rarely occur in patients with HIV as a consequence of immuno-suppression and more frequently presents as extra pulmonary manifestations [45].
Viral pneumonia: Cyto Megalo Virus (CMV) is the most common viral infection in AIDS patients. AIDS-related lymphoma and unclear interstitial pneumonia may become impersonator of PJP. In those cases, serum β-D-glucan examination and PCR for Pneumocystis should be valuable for the differential diagnosis [46].
Complications
ARDS, Lymphadenopathy, Pancytopaenia, Respiratory failure [47].
DIAGNOSIS
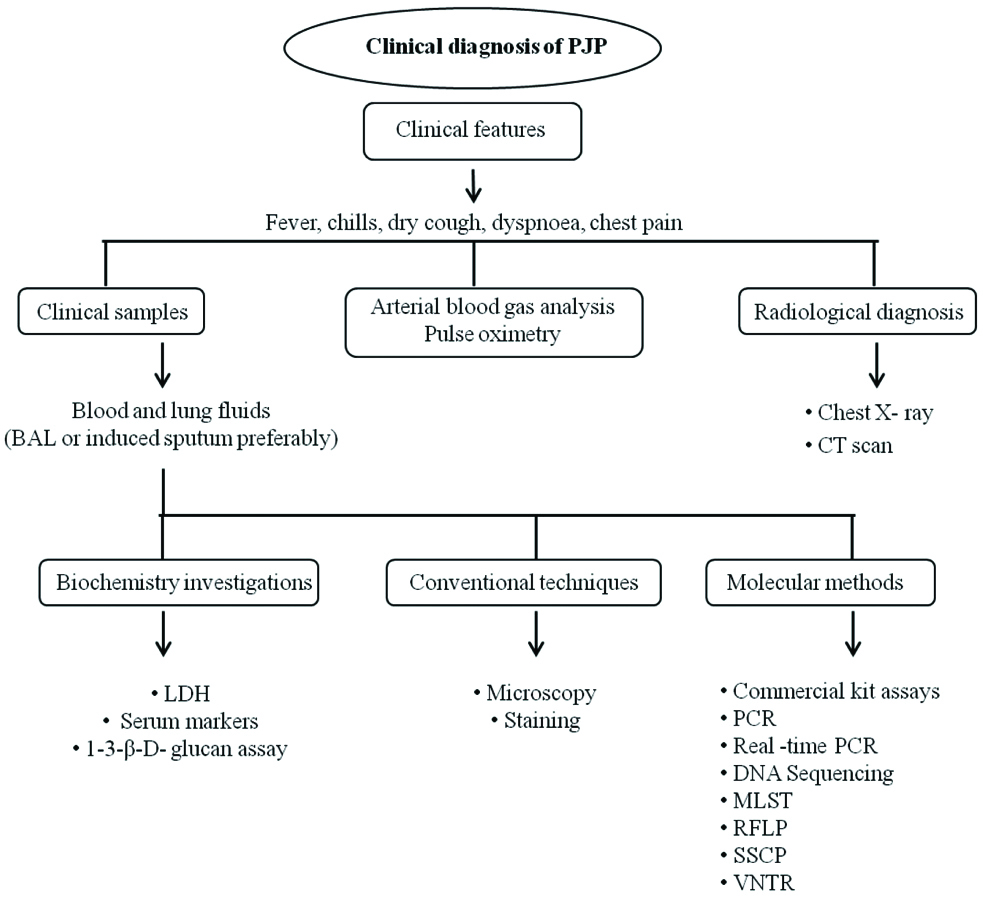
Multiple factors play a role in diagnosis of PJP and may consists of clinical features, patient risk factors, disease status, chest radiograph, chest Computed Tomography (CT), Lactate dehydrogenase LDH evaluation, other investigations and lung biopsy [47,48].
LDH Evaluation
Serum LDH levels are generally increased in PJP infection. 90% of HIV patients have shown high range of serum LDH levels. Increased serum LDH levels are seen in immuno-compromised people without HIV infection. Other various factors also may cause to raise the levels in their body. As a result, LDH evaluation is not a confirmed diagnosis of PJP [6].
S-Adenosylmethionine (AdoMet) Technique
P. jirovecii cannot synthesise S-Adenosylmethionine (AdoMet) molecule. AdoMet levels are decreased in patients with PJP infection. Measurement of S-Adenosylmethionine plasma concentrations could give to a new method for PJP diagnosis and also helpful in patient treatment [6,48].
Chest Radiography
Based on the clinical findings and severity of the illness, clinicians refer chest radiography to know the status of the disease in patients. Early mild disease condition, normal chest radiographic findings may be normal. Most of the PJP cases have shown abnormal chest radiographic findings. Chest radiography has shown diffuse bilateral infiltrates predominantly peri-hilar distribution in PJP patients. Other radiographic findings consist of pneumothorax, patchy asymmetric infiltrates, pneumatocele and fine reticular interstitial changes [47].
Computed Tomography (CT): Significant radiology findings of Pneumocystis were not seen in chest radiography, but patient’s clinical symptoms have shown infection of Pneumocystis. When chest X-ray is negative, High Resolution Computed Tomography (HRCT) is very helpful to identify considerable findings. In HIV patients, HRCT is useful to identify findings specifically. CT is the most sensitive radiology method compared to chest X-ray. In patients with Pneumocystis infection, CT scan reveals bilateral patchy ground glass appearance. Granular, reticular and cystic lesions are less common features seen in patients with PJP [49].
Arterial Blood Gas (ABG) analysis: An ABG analysis is useful marker for evaluating the severity of the PJP infection. ABG level should be measured for the probable adjunctive corticosteroid therapy in patients with hypoxic condition. Usually Alveolar-arterial (A-a) oxygen gradient is raised in PJP infection [4].
Pulmonary Function Tests
Spirometry, Diffusing Capacity for Carbon Monoxide (DLCO)
Clinical Samples:P. jirovecii is a lung pathogen. Lung exudates are used for diagnosis of PJP. Clinical samples such as Sputum, Induced sputum, BAL, Pleural fluid and Lung tissue are used for identification of Pneumocystis infection.
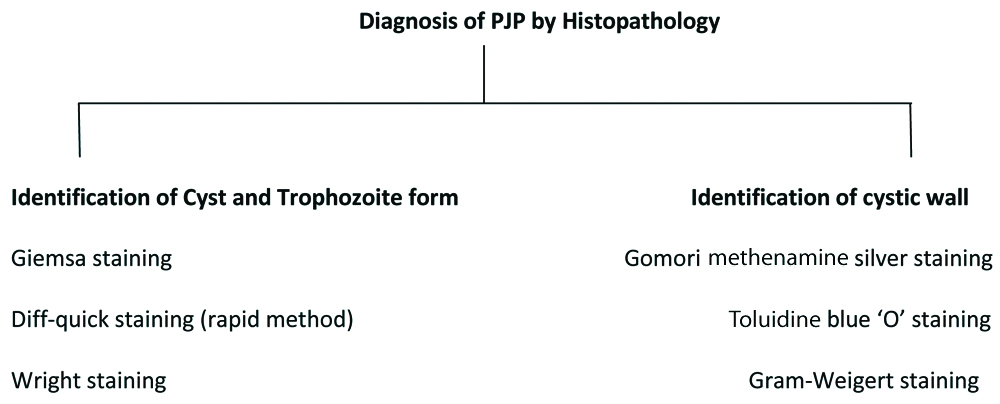
Staining: Different stains such as Methenamine silver, Calcoflour white, Toluidine blue-O, Acridine orange, Geimsa staining and Diff-Quik staining are used for identification of Pneumocystis. Immunofluorescence (IF) staining is rapid, easy to perform, most sensitive and specific method for detection of Pneumocystis compared to other conventional methods [38,50]. This staining is very helpful to the patients with low organism load [Table/Fig-3].
Diagnosis of PJP by Histopathology.
Serology
Serum markers: Krebs von den Lungen-6 (KL-6) is a mucin-like glycoprotein with high-molecular-weight firmly expressed on Type-2 alveolar pneumocytes and bronchiolar epithelial cells. Levels of KL-6 in serum are a responsive indicator of different categories of interstitial pneumonitis. These levels are high in HIV patients with PJP compared to non-HIV group of patients [51].
Using recombinant antigens of P. jiroveci and immuno-enzymatic or immuno-blotting assays have potential application in diagnosis of PJP [52].
In-vitro Cultivation
In-vitro culture system is not yet developed completely. Short time efficient in-vitro methods promote the production of infectious organisms in short period of time. Cell lines like Mink lung cell line Mv 1, human lung fibroblast line HEL and Human lung carcinoma cell line A 549 were usually used for Pneumocystis culture and conventional stains, antibodies can be used to identify them [53]. In 2014, first success was achieved by using a three-dimensional air-liquid interface culture system formed by CuFi-8 respiratory epithelial cell line. This is used for cultivation and propagation of P. jirovecii directly from the BAL sample [54].
Recent Advances in Diagnosis by Molecular Methods
Molecular methods have been developed for diagnosis of PJP. PCR assays are most sensitive and specific methods useful for detection of P. jirovecii from infected individuals, recurrent PJP patients. PCR assays can be helpful for estimation of prevalence of disease as well as epidemiological purposes. Immuno-compromised individuals without HIV may have low number of pathogen in their lung compared to HIV positive patients. Low number of pathogen burden and patients under chemoprophylaxis may decrease the sensitivity in microscopic methods, in such cases PCR assays are very helpful tools for confirmatory diagnosis [55,56]. Commonly used clinical samples are BAL, sputum, induced sputum, oral washes and pleural fluid. Different types of PCR assays can be used for different gene targets for detection of Pneumocystis. Optimised single round touchdown PCR have been used for routine purposes. Nested PCR increases the sensitivity of the test [55].
New Diagnostic methods of P.jirovecii: Commercial kits like Myc Assay, AmpliSens, Bio-Evolution PCR, Fast Track Diagnostics (FTD), to detect P. jirovecii were studied by different researchers [57-59] by comparing with conventional methods of detection.
Following are few molecular typing methods for detecting P. jirovecii [Table/Fig-4] [60]. A flowchart has been devised for diagnosis of the PJP infections [Table/Fig-5] [56,61].
Molecular typing methods [56,61].
| Sl. No | Method |
|---|
| 1. | Single-locus Sanger DNA sequencing: | This is the most common method for single-locus typing of P. jirovecii. This method is accommodating to recognise all well-known or potentially novel sequence variants in the target areas. Still Sanger sequencing is a good typing method compared to other newer methods because of attractive option in many circumstances and also with fast and inexpensive commercial sequencing services. |
| 2. | Multilocus sequence typing (MLST) | MLST involved amplified PCR product followed by DNA sequencing of a number of genes. Almost the entire recognised genetic markers for P. jirovecii have been estimated for their attainable to develop an MLST system. Although it is expensive and labour-intensive to amplify and sequence entity loci from individual patients, an effort has been ready to conquer this negative aspect by achieving whichever DNA pooling approaches or real-time amplification of many loci followed by single-base conservatory analysis. These approaches have possible for high-throughput relevance but have not been assessed by different laboratories. |
| 3. | Restriction fragment length polymorphism analysis (RFLP) | This is the most accepted method since 1980s and early 1990s to until now. This method is commonly used for typing of numerous organisms. Advantages of this method consist of in-expensive instruments, less time processing (without hybridization) and a high sensitivity (with hybridization). RFLP method is usually recognised for DHPS gene mutations of P. jirovecii. Newly, RFLP was modified to identify polymorphisms of the P. jirovecii msg repertoire. |
| 4. | Single-strand confirmation polymorphism analysis (SSCP) | SSCP is a quite easy and convenient method to distinguish nucleotide variations within and between amplicons (~100 to 500 bp). Mainly SSCP is useful for typing of P. jirovecii, its capability to discriminate various sequences in patients with co-infections and also possible to done for quick screening of huge numbers of samples per day. |
| 5. | Variable-number tandem-repeat (VNTR) analysis: | Unlike typing methods, this method is helpful to detect nucleotide substitutions or indels in non-repetitive loci. This investigation is to be dependent on enumerated the repeat copy numbers of short tandem repeats, furthermore called as microsatellites. Highest sensitivity is the main advantage of this method for detection of a small population in mixed populations of P. jirovecii and which can be observed in up to 92% of patients with PJP. |
| 6. | DNA Sequence Analysis | Commonly molecular typing studies and biodiversity of Pneumocystis is done by direct sequence analysis. The mitochondrial large subunit ribosomal RNA locus (mt LSU rRNA), dihydropteroate synthase (DHPS) gene Internal Transcribed Spacer (ITS) regions of the nuclear rRNA operon are helpful for molecular epidemiology applications compared to Superoxide dismutase (SODA) gene loci, thymidylate synthase (TS), EPSP synthase domain of the multi-functional arom gene and the mitochondrial small subunit ribosomal RNA (mt SSU rRNA). These are not highly beneficiary, because of low sequence divergence of Pneumocystis [56,61]. |
Flow chart for diagnosing PJP.
Treatment/Prevention
Treatment of PJP infection is based on the severity of the illness and diagnosis. Evidence based guidelines should be followed for giving proper treatment. Common antifungal drugs are not effective for PJP infection. PJP should be treated with prescribed medicine. If Treatment is not given, the patient will die with PJP infection. Drug of choice for PJP infection is Trimethoprim-Sulfamethoxazole (TMP-SMX), also known as co-trimoxazole. TMP-SMZ is highly effective drug compared to other drugs because of good penetration, fast clinical response and low cost. TMP-SMZ is a first line medication in both HIV and Non-HIV individuals as a 21 days course.
Treatment in HIV Patients
Mild to Moderate disease: oral medication, 3 divided doses of TMP 15 to 20 mg/kg/day and SMZ 75 to 100 mg/kg/day.
Moderate to severe cases: Intravenous (IV) administration of TMP 15 to 20 mg/kg/day and SMZ 75-100 mg/kg/day for every 6 to 8 hours [3]. Side effects are fever and rash [39]. Intensity of allergies increased by prolonged usage of drug. Alternative drug regimen can be used when TMP-SMZ side effects are severe.
Second Line Drugs for Severe Cases
Clindamycin and Primaquine: In mild and moderate diseased condition, clindamycin and primaquine are good alternative choice for treatment of PJP.
Pentamidine is the most preferred alternative drug regimen to TMP-SMZ [62,63]. Intravenous pentamidine is given at least 14-21 days as a dose of 4 mg/kg/day [64].
Second Line Drugs for Moderate or Mild Disease
Dapsone is another alternative drug for TMP-SMZ for treating PJP infection. Daily dosage of 100 mg plus trimethoprim (15 mg daily) should be administered [65].
Atovaquone: Another drug regimen for PJP infection. Daily dose of Atovaquone is 1500 mg (twice a day 750 mg), given orally with food. Atovaquone is a good alternative choice for the patients who cannot tolerate TMP-SMZ and dapsone. It has a good tolerating capacity but response of this drug is poor and also expensive [66]. This drug can be used in patients who are intolerant to TMP-SMZ and pentamidine. It is less effective compared to the TMP-SMZ drug regimen.
Haematological patients: Daily dose of TMP-SMZ 15-20 mg/kg TMP; 75-100 mg/kg SMZ. Second line drugs -Daily doses of Primaquine 30 mg and clindamycin 600 mg thrice. Daily dose of Pentamidine IV 4 mg/kg. Daily dose of Atovaquone 750 mg twice or thrice. As per ECIL guidelines, currently high dose of TMP-SMZ over two week’s period is a treatment of choice in PJP patients with haematological malignancies [67].
Solid organ transplantation patients: Daily dose of TMP-SMZ 15-20 mg/kg; 75-100 mg/kg) should be received by patient with every 6-8 hours time, TMP administered by IV route. Combination with daily dose of prednisolone 40-60 mg should be given twice in hypoxemic patients [68].
Recent Treatment Modalities
Treatment Modality with Caspofungin
Li H et al., reported, Caspofungin combined with Clindamycin is alternative treatment for PJP infection when treatment fails with TMP-SMZ and patients intolerant to TMP-SMZ [69].
Caspofungin with TMP-SMZ
Another recent study by Yu B et al., evaluated and reported that combined therapy of Caspofungin with TMP-SMZ is more effective than TMP-SMZ monotherapy in renal transplant patients [70].
Recent study from Japan showed Sulfasalazine is a better treatment choice in Rheumatoid Arthritis (RA) patients due to high preventive effect of drug against PJP [71].
Retroviral Therapies Interfere with Survival Rate of the Patients Infected with PJP
Immune-reconstitution, anti-retroviral therapy is one of the effective way of avoiding PJP in individuals alive with HIV. Anti PJP and anti-retroviral therapies comprise additive or synergistic toxicities. It may lead to delay in the instigation of anti-retroviral therapy until after beginning anti-PCP therapy or in a few cases until after the end of anti-PJP therapy. AIDS succession mortality can be diminished in people with acute opportunistic infections by initiating early antiretroviral therapy. This study showed both therapies were not linked with the side-effects as well as decrease in the effectiveness of antiretroviral therapy. Eventually study concluded that Anti Retro Viral therapies interfere with a reduction in AIDS progression and increase in survival rate [72].
Adjuvant Corticosteroids Affect the Course of Disease in Immuno-compromised Patients
In HIV patients: Early involvement of corticosteroids in AIDS patients with severe PJP infection give results in an impressive survival benefit and reduced the frequency of respiratory failure. Early adjuvant corticosteroid therapy at least 7 days with severe PJP may reduce intensive care unit stay. Moderate to severe PJP, adjunctive corticosteroids initiated at the time of PJP therapy prevented the early decline in oxygenation (arterial-alveolar difference >35 mmHg or an arterial oxygen pressure <70 mmHg) may lead to respiratory failure and death. In children with HIV positive condition, usage of short course corticosteroids may be helpful for management of PJP. In solid organ transplant patients, usage of corticosteroids may be a risk factor and developing PJP. Widely used corticosteroids for management of haematological malignancies may be a risk factor for PJP infection [65,73].
Prophylaxis
Evidence based guidelines play a major role for prophylaxis in certain individuals. Primary prophylaxis should be given for PJP patients with immuno-suppressive conditions including haematological malignancy, AIDS, congenital immunodeficiency, organ transplantation. TMP-SMZ is given as an early treatment prophylaxis in patients with PJP. If side effects are severe with TMP-SMZ, other drugs include Pentamidine, Trimetrexate, Atovaquone, Clindamycin and Primaquine combined with Leucovorin, Dapsone and Caspofungin combined with Clindamycin can be given as alternative drug regimen to the patients with PJP infection.
Indications: Primary prophylaxis is indicated to the HIV patients with CD4 count less than 200 cells/μL [5], oropharyngeal candidiasis and patients with history of other opportunistic infections. Secondary prophylaxis is indicated for patients with a history of PJP.
Clinicians can stop the primary and secondary treatment prophylaxis in patients which had responded to (HAART) with a raise of CD4 count >200 cells/μL. CD4 count monitoring should be crucial for every three months. If CD4 count is <200 cells/μL, prophylaxis should be restarted. This is a safety practice proven by studies. This type of treatment prophylaxis can be helpful to decrease the usage of drug regimen, side effects of drug and variety of drug challenging pathogens [74,75].
Drug Resistance and Mutations
Drug resistance is the common problem usually observed in microorganisms. Earlier to the HIV epidemic in developing countries, usage of TMP-SMZ is rare in patients due to toxic effect. Very low drug resistance was reported in those countries [76]. Later TMP-SMZ used as common drug regimen for treatment of PJP. Extensive utilisation of TMP-SMZ and Dapsone in HIV and other immuno-compromised individuals can lead to Sulfa (Sulfonamide or Sulfone) drug resistance. As it is difficult to culture Pneumocystis, drug susceptibility testing is not possible.
Wide spread and long term usage of PJP prophylaxis can lead to rising new cases of DHPS mutant strains. The spread of resistant mutants due to genetic mutations and other possible reason may be person-to-person transmission of P. jirovecii infection [77,78]. A lot of single base polymorphisms were reported in DHPS of P. jirovecii. Substrate binding was affected by these mutations and exhibit resistance to dapsone and sulphonamide. Mutations occurred in cytochrome b gene exhibit atovaquone resistant P. jirovecii. Survival rate does not completely depend upon the mutated P. jirovecii strains and may be other factors can play a role in HIV patients [79,80]. Evaluation of drug resistance is complicated in HIV patients and finally further advance research is required for strategies development as well as to stop the further increase of resistant strains.
Recent study from Delhi reported frequency of mutations in the DHPS gene of P. jirovecii isolates. A novel mutant strain was reported in 3 cases (25.0%) among 12 cases of infected patients and no mutations in remaining 9 cases. Aforesaid 9 cases, responded very well to the treatment. Therefore, novel DHPS mutant strain is associated with drug resistance and mortality [35].
Very fewer studies conducted on genetic variations in P. jirovecii Dihydrofolate reductase (DHFR) gene. Almost 12 of the studies reported, no DHFR mutations were observed. In dissimilarity, about 6 studies were reported, wide-ranging mutations was seen at more than 30 amino acid positions. Through in-vitro testing, recombinant P. jirovecii of DHFR enzymes comprised known mutations. Regarding this six DHFR variants were resistant to TMP and may leads to clinical resistance. Few studies conducted on atovaquone resistance. It targets mitochondrial gene cytochrome b (COB). More than 70 isolates revealed different mutations by sequence analysis with only 2 present in more than one isolate. These three drug targets DHPS, DHFR, and COB were also detected in PJP patients with no previous contact to the concerned drugs. These patients may spread mutant P. jirovecii strains to other persons. These mutations concurrently with identical nucleotide changes in these genes may be helpful as indicators in epidemiological purposes [60].
Genetic Influences of P. jirovecii
Genetic diversity plays a key role in adapting to changing environments, for the survival of a species. Cisse OH et al., investigated the diversity and demographic histories of natural populations of Pneumocystis, communicate a disease to humans, rats, and mice [81]. They have collected infected tissues from various geographical settings and carried out whole-genome and large scale multilocus sequencing. They reported rate of evolution, range of population and levels of genetic diversity. This study revealed high rate of infection and natural populations maintain an elevated level of genetic variation in spite of low levels of recombination. Finally, this study results give a dynamic outlook of the evolution of Pneumocystis populations and improve indulgent of its transmission [81].
Duggal P et al., examined the effects of a novel non-conservative acidic to basic alter in the third codon of the extracellular domain of CXCR6 on point in time to death after a proven diagnosis of PJP [82]. This study concluded that there is an association among genotype CXCR6 and progress delayed from PJP to death among African-Americans with HIV disease. This study proposed with the aim of CXCR6 may play a role in stage HIV-1 infection and may change the progression to fatality after early infection with PJP [82].
Risk of Evaluation and Outcome in Solid Organ Transplant (SOT) Patients
As per American Society of Transplantation Infectious Diseases Community of Practice guidelines 2019, P. jirovecii infection may develop through airborne route or reactivation of previous infection in organ transplant patients. Generally PJP have shown highest risk in first 6 months after organ transplantation. Better prophylaxis drug is TMP-SMZ. No prophylaxis may increase the risk of PJP. Lower death rate in SOT identified as an independent variable associated with a lower death rate and similar to that of AIDS. A deferred diagnosis, resultant in a delay in the beginning of a modified PCP treatment, can contribute in a poor outcome where as the time from admission to beginning of PCP treatment is an independent predictor of death being older, needing oxygen or invasive mechanical ventilation on admission signifies a poor prognosis. A World Health Organisation (WHO) performance standing over 2, an uncontrolled of the underlying infection, a high temperature, hypoalbuminemia, shock, and clinical worsening by day 5 are also associated with raised mortality. Pulmonary co-infections with CMV or herpes simplex virus and an elevated neutrophil count in a BAL sample are also correlated with more severe hypoxemia and higher mortality rates [83,84].
Implications in Chronic Lymphocytic Leukaemia Patients
As per ECIL guidelines, no antifungal treatment is needed for Chronic Lymphocytic Leukaemia. Patients with prolonged neutropaenia (more than 6 months) elderly, advanced and impassive disease necessitate it [67].
Conclusion
PJP remains a still major health problem in both HIV and other immuno-compromised individuals. Improper treatment for PJP infection may lead to mortality. HIV cases with HAART may reduce the risk of PJP. Mortality is associated with PJP in both HIV and non-HIV patients. The rate of infection as well as mortality rates was increasing year by year. Major problem in AIDS patients with Pneumocystis infection leads to increasing death rate due to failure of drug therapy. Finally, further advance research is required for strategies development as well as to stop further increase of resistant strains. New diagnostic methods and usage of non-invasive respiratory specimens such as oral washes for diagnosis of PJP should be expanded. PCR is the most sensitive method compared to other conventional and serological methods. Using PCR and Sequence analysis can be helpful for estimation of prevalence of disease as well as epidemiological purposes. Following evidence based guidelines will be helpful for giving proper treatment and can reduce the improper usage of drugs and decrease drug resistance. Conducting programmes to public, regarding disease will be helpful for patient management particularly in HIV people suffering with opportunistic infections. There is an urgent need to develop newer drugs and vaccines to eradicate the PJP disease burden.
[1]. Ricciardi A, Gentilotti E, Coppola L, Maffongelli G, Cerva C, Malagnino V, Infectious disease ward admission positively influences P. jiroveci pneumonia (PJP) outcome: A retrospective analysis of 116 HIV-positive and HIV-negative immunocompromised patientsPLoS ONE 2017 12(5):e017688110.1371/journal.pone.017688128505159 [Google Scholar] [CrossRef] [PubMed]
[2]. Mecoli CA, Saylor D, Gelber AC, Christopher-Stine L, Pneumocystis jirovecii pneumonia in rheumatic disease: A 20-year single-centre experienceClin. Exp. Rheumatol 2017 35(4):671-73. [Google Scholar]
[3]. Truong J, Ashurst JV, Pneumocystis (Carinii) Jiroveci Pneumonia. [Updated 2019 Feb 22]In: StatPearls [Internet] 2019 Jan- Treasure Island (FL)StatPearls PublishingAvailable from: https://europepmc.org/books/NBK482370;jsessionid=100D61CBCF6B7F6B061C2FD3DB0A60F9 [Google Scholar]
[4]. Shelley A Gilroy. Pneumocystis jiroveci Pneumonia (PJP) [Updated 2019 Apr 24]. Available from: https://emedicine.medscape.com/article/225976-overview [Google Scholar]
[5]. Kaplan JE, Hanson D, Dworkin MS, Frederick T, Bertolli J, Lindegren ML, Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapyClin Infect Dis 2000 30(Suppl 1):S5-14.10.1086/31384310770911 [Google Scholar] [CrossRef] [PubMed]
[6]. George MP, Gingo MR, Morris A. Pneumocystis (carinii) jirovecii. Available from http://www.antimicrobeorg/new/f11asp#top. Accessed on 04.06.2019 [Google Scholar]
[7]. Amber KT, Balancing the risks and benefits of prophylaxis: a reply to “Pneumocystis jiroveci pneumonia in patients treated with systemic immunosuppressive agents for dermatologic conditions”Int J Dermatol 2017 56(1):e4-e5.10.1111/ijd.1339527653175 [Google Scholar] [CrossRef] [PubMed]
[8]. Fillâtre P, Revest M, Belaz S, Robert-Gangneux F, Zahar JR, Roblot F, [Pneumocystosis in non-HIV-infected immunocompromised patients]Rev Med Interne 2016 37(5):327-36.10.1016/j.revmed.2015.10.00226644039 [Google Scholar] [CrossRef] [PubMed]
[9]. Antinori A, Maiuro G, Pallavicini F, Valente F, Ventura G, Marasca G, Prognostic factors of early fatal outcome and long-term survival in patients with Pneumocystis carinii pneumonia and acquire immunodeficiency syndromeEur J Epidemiol 1993 9:183-89.10.1007/BF001587898100199 [Google Scholar] [CrossRef] [PubMed]
[10]. Stringer JR, Beard CB, Miller RF, Wakefield AE, A new name (Pneumocystis jirovecii) for Pneumocystis from humansEmerg Infect Dis 2002 8:891-96.10.3201/eid0809.02009612194762 [Google Scholar] [CrossRef] [PubMed]
[11]. Ioannidis JP, Cappelleri JC, Skolnik PR, Lau J, Sacks HS, A meta-analysis of the relative efficacy and toxicity of Pneumocystis Carinii prophylactic regimensArch Intern Med 1996 156:177-88.10.1001/archinte.1996.004400200810108546551 [Google Scholar] [CrossRef] [PubMed]
[12]. Centers for Disease Control (CDC)Pneumocystis pneumonia--Los AngelesMorb Mortal Wkly Rep 1981 30:250-52. [Google Scholar]
[13]. Masur H, HIV-Related opportunistic infections are still relevant in 2015Top Antivir Med 2015 23(3):116-19. [Google Scholar]
[14]. Rey A, Losada C, Santillán J, Fiorentini F, Schiaffino M, Peroni HJ, Pneumocystis jiroveci infection in patients with and without HIV: A comparisonRev Chilena Infectol 2015 32:175-80.10.4067/S0716-1018201500030000626065450 [Google Scholar] [CrossRef] [PubMed]
[15]. Solano L MF, Alvarez Lerma F, Grau S, Segura C, Aguilar A, Pneumocystis jirovecii pneumonia: Clinical characteristics and mortality risk factors in an Intensive Care UnitMed Intensiva 2015 39:13-19.10.1016/j.medin.2013.11.00624485532 [Google Scholar] [CrossRef] [PubMed]
[16]. Buchacz K, Lau B, Jing Y, Bosch R, Abraham AG, Gill MJ, North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Incidence of AIDS-Defining Opportunistic Infections in a Multicohort Analysis of HIV-infected Persons in the United States and Canada, 2000-2010J Infect Dis 2016 214:862-72.10.1093/infdis/jiw085 [Google Scholar] [CrossRef]
[17]. de Armas Rodriguez Y, Wissmann G, Muller AL, Pneumocystis jirovecii pneumonia in developing countriesParasite 2011 18:219-28.10.1051/parasite/201118321921894262 [Google Scholar] [CrossRef] [PubMed]
[18]. Weverling GJ, Mocroft A, Ledergerber B, Kirk O, Gonzales-Lahoz J, d’Arminio Monforte A, Discontinuation of Pneumocystis carinii pneumonia prophylaxis after start of highly active antiretroviral therapy in HIV- 1 infection. EuroSIDA Study GroupLancet 1999 353:1293-98.10.1016/S0140-6736(99)03287-0 [Google Scholar] [CrossRef]
[19]. Hughes WT, Current issues in the epidemiology, transmission and reactivation of PneumocystiscariniiSemin Respir Infect 1998 13:283-88. [Google Scholar]
[20]. Malin AS, Gwanzura LK, Klein S, Robertson VJ, Musvaire P, Mason PR, Pneumocystiscarinii pneumonia in ZimbabweLancet 1995 346:1258-61.10.1016/S0140-6736(95)91862-0 [Google Scholar] [CrossRef]
[21]. Fisk DT, Meshnick S, Kazanjian PH, Pneumocystis carinii pneumonia in patients in the developing world who have acquired immunodeficiency syndromeClin Infect Dis 2003 36:70-78.10.1086/34495112491205 [Google Scholar] [CrossRef] [PubMed]
[22]. Singh YN, Singh S, Rattan A, Ray JC, Sriniwas TR, Kumar A, Pneumocystis carinii infection in patients of AIDS in IndiaJ Assoc Physicians India 1993 41:41-42. [Google Scholar]
[23]. Mirdha BR, Guleria R, Comparative yield of different respiratory samples for diagnosis of Pneumocystis carinii infections in HIV seropositive and sero-negative individuals in IndiaSouth-East Asian J Trop Med Pub Health 2000 31:473-77. [Google Scholar]
[24]. Merchant RH, Oswal JS, Bhagwat RV, Karkare J, Clinical profile of HIV infectionIndian Pediatr 2001 38(3):239-46. [Google Scholar]
[25]. Kumarasamy N, Vallabhaneni S, Flanigan TP, Mayer KH, Solomon S, Clinical profile of HIV in IndiaIndian J Med Res 2005 121:377-94.10.1097/01.qai.0000176591.06549.de16249715 [Google Scholar] [CrossRef] [PubMed]
[26]. Rajagopalan N, Suchitra JB, Shet A, Khan ZK, Martingarcia J, Nonnemacher MR, Mortality among HIV-Infected patients in resource limited settings: A case controlled analysis of inpatients at a community care centerAm J Infect Dis 2009 5:219-24.10.3844/ajidsp.2009.219.22420204076 [Google Scholar] [CrossRef] [PubMed]
[27]. Shahapur PR, Bidri RC, Recent trends in the spectrum of opportunistic infections in human immunodeficiency virus infected individuals on antiretroviral therapy in South IndiaJ Nat Sc Biol Med 2014 5:392-96.10.4103/0976-9668.136200 [Google Scholar] [CrossRef]
[28]. Chawla K, Martena S, Gurung B, Mukhopadhyay C, Varghese GK, Bairy I, Role of PCR for diagnosing Pneumocystis jirovecii pneumonia in HIV-infected individuals in a tertiary care hospital in IndiaIndian J Pathol Microbiol 2011 54:326-29.10.4103/0377-4929.8162421623083 [Google Scholar] [CrossRef] [PubMed]
[29]. Ramesh K, Gandhi S, Rao V, Clinical profile of human immunodeficiency virus patients with opportunistic infections: A descriptive case series studyInt J App Basic Med Res 2015 5:119-23.10.4103/2229-516X.15716626097820 [Google Scholar] [CrossRef] [PubMed]
[30]. Jairam A, Dassi M, Chandola P, Lall M, Mukherjee D, Hooda AK, Pneumocystis jiroveci outbreak in a renal transplant center: Lessons learntIndian J Nephrol 2014 24(5):276-79.10.4103/0971-4065.13298725249715 [Google Scholar] [CrossRef] [PubMed]
[31]. Kaur R, Panda PS, Dewan R, Profile of pneumocystis infection in a tertiary care institute in North IndiaIndian J Sex Transm Dis AIDS 2016 37(2):143-46.10.4103/0253-7184.18550127890947 [Google Scholar] [CrossRef] [PubMed]
[32]. Deodhar D, Koshy JM, John M, Oberoi A, Clinical profile of pneumocystis jirovecii infection- A comparative studyJ Assoc Physicians India 2018 66(1):28-31. [Google Scholar]
[33]. Jeswani J, Godara S, Bhagat C, Risk factors, clinical manifestations, and outcomes of Pneumocystis jirovecii infection in post-renal transplant recipientsJ Egypt Soc Nephrol Transplant 2018 18:112-15. [Google Scholar]
[34]. Shilpa Andgi A, Clinical profile of opportunistic infections in HIV seropositive patients attending tertiary centre, Raichur, IndiaInt J Adv Med 2018 5(6):1369-73.10.18203/2349-3933.ijam20184738 [Google Scholar] [CrossRef]
[35]. Singh Y, Mirdha BR, Guleria R, Kabra SK, Mohan A, Chaudhry R, Novel dihydropteroate synthase gene mutation in Pneumocystis jirovecii among HIV-infected patients in India: Putative association with drug resistance and mortalityJ Glob Antimicrob Resist 2019 17:236-39.10.1016/j.jgar.2019.01.00730658203 [Google Scholar] [CrossRef] [PubMed]
[36]. Kumarasamy N, Venkatesh KK, Devaleenol B, Poongulali S, Yephthomi T, Pradeep A, actors associated with mortality among HIV-infected patients in the era of highly active antiretroviral therapy in southern IndiaInt J Infect Dis 2010 14(2):e127-31.10.1016/j.ijid.2009.03.03419632872 [Google Scholar] [CrossRef] [PubMed]
[37]. Udwadia ZF, Doshi AV, Bhaduri AS, Pneumocystis carinii pneumonia in HIV infected patients from MumbaiJ Assoc Physicians India 2005 53:437 [Google Scholar]
[38]. Prevost MC, Escamilla, R, Aliouat, EM, Cere, N, Coudert Pneumocystosis pathophysiologyFEMS Immunol Med Microbiol 1998 22:123-28.10.1016/S0928-8244(98)00069-8 [Google Scholar] [CrossRef]
[39]. Pneumocystis pneumonia. Fungal Diseases. CDC https://www.cdc.Gov/fungal/diseases/pneumocystis-pneumonia [Google Scholar]
[40]. Sheikholeslami MF, Sadraei J, Farnia P, Forozandeh Moghadam M, Emadi Kochak H, Co-infection of Mycobacterium tuberculosis and Pneumocystis jirovecii in the Iranian patients with human immunodeficiency virusJundishapur J Microbiol 2015 8(2):e1725410.5812/jjm.1725425825645 [Google Scholar] [CrossRef] [PubMed]
[41]. Nethathe G, Patel N, Survival after Pneumocystis jirovecii pneumonia requiring ventilation: A case reportSouth Afr J HIV Med 2016 17(1):47410.4102/sajhivmed.v17i1.47429568616 [Google Scholar] [CrossRef] [PubMed]
[42]. Govender S, du Plessis SJ, Ocana GS, Chalkley LJ, Prevalence of Pneumocystis jirovecii and Mycoplasma pneumoniae in patients presenting with pneumonia at hospitals in Port ElizabethSouthern African Journal of Epidemiology and Infection 2008 23(2):21-24.10.1080/10158782.2008.11441309 [Google Scholar] [CrossRef]
[43]. Sundsted KK, Syed H, Burton MC, 69-year-old woman with dyspnea and cough productive of white sputumMayo Clin Proc 2011 86(12):1225-28.10.4065/mcp.2011.013122134941 [Google Scholar] [CrossRef] [PubMed]
[44]. Simkins J, Corrales-Medina V, Symes S, Dickinson G, Pulmonary embolism in patients with acquired immunodeficiency syndrome presenting with clinical picture of Pneumocystis jiroveci pneumonia: report of two casesScand J Infect Dis 2007 39(6-7):634-36.10.1080/0036554060114850917577835 [Google Scholar] [CrossRef] [PubMed]
[45]. čurić K, Poljak M, Ihan A, Tomažič J, Very recent HIV infection accompanied by Pneumocystis jirovecii pneumonia and Mycobacterium avium complex immune reconstitution inflammatory syndrome: A case reportActa Dermatovenerol Alp Pannonica Adriat 2016 25(3):57-58.10.15570/actaapa.2016.1627695869 [Google Scholar] [CrossRef] [PubMed]
[46]. Tasaka S, Pneumocystis pneumonia in human immunodeficiency virus-infected adults and adolescents: Current concepts and future directionsClin Med Insights Circ Respir Pulm Med 2015 9(Suppl 1):19-28.10.4137/CCRPM.S2332426327786 [Google Scholar] [CrossRef] [PubMed]
[47]. Carmona EM, Limper AH, Update on the diagnosis and treatment of PneumocystispneumoniaTherapeutic Advances in Respiratory Disease 2011 :41-59.10.1177/175346581038010220736243 [Google Scholar] [CrossRef] [PubMed]
[48]. Skelly M, Hoffman J, Fabbri M, Holzman RS, Clarkson AB Jr, Merali S, S-adenosylmethionine concentrations in diagnosis of Pneumocystis carinii pneumoniaLancet 2003 361:1267-68.10.1016/S0140-6736(03)12984-4 [Google Scholar] [CrossRef]
[49]. Hidalgo A, Falcó V, Mauleón S, Andreu J, Crespo M, Ribera E, Accuracy of high-resolution CT in distinguishing between Pneumocystis carinii pneumonia and non-Pneumocystis carinii pneumonia in AIDS patientsEur Radiol 2003 13(5):1179-84.Epub 2002 Sep 25 [Google Scholar]
[50]. Touhali IS, Ibrahim AAF, Dawood HN, Conventional methods for the diagnosis of pneumocystis jiroveci in immunocompromised Iraqi patientsIraqi JMS14:80-87. [Google Scholar]
[51]. Tasaka S, Tokuda H, Recent advances in the diagnosis of Pneumocystis jirovecii pneumonia in HIV-infected adultsExpert Opin Med Diagn 2013 7(1):85-97.10.1517/17530059.2012.72208023530845 [Google Scholar] [CrossRef] [PubMed]
[52]. Tomás AL, Cardoso F, Esteves F, Matos O, Serological diagnosis of pneumocystosis: production of a synthetic recombinant antigen for immunodetection of Pneumocystis jiroveciiSci Rep 2016 6:3628710.1038/srep3628727824115 [Google Scholar] [CrossRef] [PubMed]
[53]. Contini C, Mastrantoni S, Romani R, Cultera R, Delia S, Evidence of Pneumocystis carinii in cell line cultures infected with peripheral blood mononuclear cells isolated from AIDS patients with P. carinii pneumoniaJ Med Microbiol 1995 42:394-98.10.1099/00222615-42-6-3947791202 [Google Scholar] [CrossRef] [PubMed]
[54]. Lewis White P, Backx M, Barnes RA, Diagnosis and management of Pneumocystis jirovecii infectionExpert Review of Anti-infective Therapy 2017 15(5):435-47.10.1080/14787210.2017.130588728287010 [Google Scholar] [CrossRef] [PubMed]
[55]. Helweg-Larsen J, Jensen JS, Dohn B, Benfield TL, Lundgren B, Detection of Pneumocystis DNA in samples from patients suspected of bacterial pneumonia- A case-control studyBMC Infect Dis 2002 2:28Epub 2002 Nov 2510.1186/1471-2334-2-2812445330 [Google Scholar] [CrossRef] [PubMed]
[56]. Durand-Joly I, Chabé M, Soula F, Delhaes L, Camus D, Dei-Cas E, Molecular diagnosis of Pneumocystis pneumoniaFEMS Immunol Med Microbiol 2005 45(3):405-10.10.1016/j.femsim.2005.06.00616061360 [Google Scholar] [CrossRef] [PubMed]
[57]. McTaggart LR, Wengenack NL, Richardson SE, Validation of the MycAssay Pneumocystis kit for detection of Pneumocystis jirovecii in bronchoalveolar lavage specimens by comparison to a laboratory standard of direct immunofluorescence microscopy, real-time PCR, or conventional PCRJ Clin Microbiol 2012 50(6):1856-59.10.1128/JCM.05880-1122422855 [Google Scholar] [CrossRef] [PubMed]
[58]. Sasso M, ChastangDumas E, Bastide S, Alonso S, Lechiche C, Bourgeois N, Lachaud L, Performances of four real-time PCR assays for diagnosis of Pneumocystis jirovecii pneumoniaJ Clin Microbiol 2016 54:625-30.10.1128/JCM.02876-1526719435 [Google Scholar] [CrossRef] [PubMed]
[59]. Hoarau G, Le Gal S, Zunic P, Poubeau P, Antok E, Jaubert J, Nevez G, Picot S, Evaluation of quantitative FTD- Pneumocystis jirovecii kit for Pneumocystis infection diagnosisDiagn Microbiol Infect Dis 2017 89(3):212-17.10.1016/j.diagmicrobio.2017.08.00128851493 [Google Scholar] [CrossRef] [PubMed]
[60]. Ma L, Cissé OH, Kovacs JA, A molecular window into the biology and epidemiology of pneumocystis sppClin Microbiol Rev 2018 31(3)10.1128/CMR.00009-1829899010 [Google Scholar] [CrossRef] [PubMed]
[61]. Beard CB, Roux P, Nevez G, Hauser PM, Kovacs JA, Unnasch TR, Strain typing methods and molecular epidemiology of PneumocystispneumoniaEmerg Infect Dis 2004 10(10):1729-35.10.3201/eid1010.03098115504257 [Google Scholar] [CrossRef] [PubMed]
[62]. Sattler FR, Cowan R, Nielsen DM, Ruskin J, Trimethoprim-sulfamethoxazole compared with pentamidine for treatment of Pneumocystiscarinii pneumonia in the acquired immunodeficiency syndrome. A prospective, non crossover studyAnn Intern Med 1988 109:280-87.10.7326/0003-4819-109-4-2803260759 [Google Scholar] [CrossRef] [PubMed]
[63]. Wharton JM, Coleman DL, Wofsy CB, Luce JM, Blumenfeld W, Hadley WK, Trimethoprim-sulfamethoxazole or pentamidine for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A prospective randomized trialAnn Intern Med 1986 105:37-44.10.7326/0003-4819-105-1-373521428 [Google Scholar] [CrossRef] [PubMed]
[64]. Limper AH, Know KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, American Thoracic Society Working Group. An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patientsAJRCCM 2011 183:96-128.10.1164/rccm.2008-740ST21193785 [Google Scholar] [CrossRef] [PubMed]
[65]. White PL, Price JS, Backx M, Therapy and Management of Pneumocystis jirovecii InfectionJ Fungi (Basel) 2018 4(4):12710.3390/jof404012730469526 [Google Scholar] [CrossRef] [PubMed]
[66]. Hughes W, Leoung G, Kramer F, Bozzette SA, Safrin S, Frame P, Comparison of atovaquone (566C80) with trimethoprim-sulfamethoxazole to treat Pneumocystis carinii pneumonia in patients with AIDSN Engl J Med 1993 328:1521-27.10.1056/NEJM1993052732821038479489 [Google Scholar] [CrossRef] [PubMed]
[67]. Maertens J, Cesaro S, Maschmeyer G, Einsele H, Donnelly JP, Alanio A, 5th European Conference on Infections in Leukaemia (ECIL-5), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organisation for Research and Treatment of Cancer (EORTC), the Immunocompromised Host Society (ICHS) and the European LeukemiaNet (ELN). ECIL guidelines for preventing Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipientsJ Antimicrob. Chemother 2016 71:2397-2404.10.1093/jac/dkw157 [Google Scholar] [CrossRef]
[68]. Kosaka M, Ushiki A, Ikuyama Y, Hirai K, Matsuo A, Hachiya T, A four-center retrospective study of the efficacy and toxicity of low-dose trimethoprim sulfamethoxazole for the treatment of pneumocystis pneumonia in patients without HIV infectionAntimicrob Agents Chemother 2017 61(12)10.1128/AAC.01173-1728893787 [Google Scholar] [CrossRef] [PubMed]
[69]. Li H, Huang H, He H, Successful treatment of severe Pneumocystis pneumonia in an immunosuppressed patient using caspofungin combined with clindamycin: A case report and literature reviewBMC Pulm Med 2016 16(1):14410.1186/s12890-016-0307-027835947 [Google Scholar] [CrossRef] [PubMed]
[70]. Yu B, Yang Y, Ye L, Xie X, Guo J, Comparison of caspofungin and trimethoprim-sulfamethoxazole combination therapy with standard monotherapy in patients with Pneumocystis jiroveci pneumonia following kidney transplantation: A retrospective analysis of 22 casesInt J Clin Exp Med 2017 10(1):1234-42. [Google Scholar]
[71]. Nunokawa T, Yokogawa N, Shimada K, Sugii S, Nishino J, Gosho M, Prophylactic effect of sulfasalazine against Pneumocystis pneumonia in patients with rheumatoid arthritis: A nested case-control studySemin Arthritis Rheum 2019 48(4):573-78.10.1016/j.semarthrit.2018.05.01330057321 [Google Scholar] [CrossRef] [PubMed]
[72]. Zolopa A, Andersen J, Powderly W, Sanchez A, Sanne I, Suckow Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: A multicenter randomized strategy trialPLoS ONE 2009 4:e557510.1371/journal.pone.000557519440326 [Google Scholar] [CrossRef] [PubMed]
[73]. Cooley L, Dendle C, Wolf J, Teh BW, Chen SC, Boutlis C, Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies. 2014Intern Med J 2014 44(12b):1350-63.10.1111/imj.1259925482745 [Google Scholar] [CrossRef] [PubMed]
[74]. Furrer H, Egger M, Opravil M, Discontinuation of primary prophylaxis against Pneumocystis carinii pneumonia in HIV-1-infected adults treated with combination antiretroviral therapy. Swiss HIV Cohort StudyN Engl J Med 1999 340:1301-06.10.1056/NEJM19990429340170110219064 [Google Scholar] [CrossRef] [PubMed]
[75]. Ledergerber B, Mocroft A, Reiss P, Furrer H, Kirk O, Bickel M, Eight European Study Groups. Discontinuation of secondary prophylaxis against Pneumocystis carinii pneumonia in patients with HIV infection who have a response to antiretroviral therapy. Eight European Study GroupsN Engl J Med 2001 344(3):168-74.10.1056/NEJM20010118344030211188837 [Google Scholar] [CrossRef] [PubMed]
[76]. Harris JR, Marston BJ, Sangrujee N, DuPlessis D, Park B, Cost-effectiveness analysis of diagnostic options for pneumocystis pneumonia (PCP)PLoS One 2011 6(8):e2315810.1371/journal.pone.002315821858013 [Google Scholar] [CrossRef] [PubMed]
[77]. Helweg-Larsen J, Benfield TL, Eugen-Olsen J, Lundgren JD, Lundgren B, Effects of mutations in Pneumocystis carinii dihydropteroate synthase gene on outcome of AIDS-associated P. carinii pneumoniaLancet 1999 354(9187):1347-51.10.1016/S0140-6736(99)03320-6 [Google Scholar] [CrossRef]
[78]. Kazanjian P, Armstrong W, Hossler PA, Burman W, Richardson J, Lee CH, Pneumocystis carinii mutations are associated with duration of sulfa or sulfone prophylaxis exposure in AIDS patientsJ Infect Dis 2000 182(2):551-57.10.1086/31571910915088 [Google Scholar] [CrossRef] [PubMed]
[79]. Friaza V, Morilla R, Respaldiza N, de la Horra C, Calderón EJ, Pneumocystis jiroveci dihydropteroate synthase gene mutations among colonized individuals and Pneumocystis pneumonia patients from SpainPost grad Med 2010 122(6):24-28.10.3810/pgm.2010.11.221921084778 [Google Scholar] [CrossRef] [PubMed]
[80]. Baggish AL, Hill DR, Antiparasitic agent atovaquoneAntimicrob Agents Chemother 2002 46(5):11637310.1128/AAC.46.5.1163-1173.200211959541 [Google Scholar] [CrossRef] [PubMed]
[81]. Cisse OH, Ma L, Wei Huang D, Khil PP, Dekker JP, Kutty G, Comparative population genomics analysis of the mammalian fungal pathogen pneumocystisMBio 2018 9:e00381-18.10.1128/mBio.00381-1829739910 [Google Scholar] [CrossRef] [PubMed]
[82]. Duggal P, An P, Beaty TH, Strathdee SA, Farzadegan H, Markham RB, Genetic influence of CXCR6 chemokine receptor alleles on PCP-mediated AIDS progression among African AmericansGenes Immun 2003 4(4):245-50.10.1038/sj.gene.636395012761559 [Google Scholar] [CrossRef] [PubMed]
[83]. Iriart X, Bouar ML, Kamar N, Berry A, Pneumocystis pneumonia in solid-organ transplant recipientsJ Fungi (Basel) 2015 1(3):293-331.Review10.3390/jof103029329376913 [Google Scholar] [CrossRef] [PubMed]
[84]. Fishman JA, Gans H, AST Infectious Diseases Community of Practice. Pneumocystis jiroveci in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of PracticeClin Transplant 2019 :e1358710.1111/ctr.1358731077616 [Google Scholar] [CrossRef] [PubMed]